Application of lycium barbarum glycopeptide in preparation of medicine for preventing or treating xerophthalmia
A dry eye syndrome and glycopeptide technology, applied in the field of biomedicine, can solve the problem of prevention or treatment of dry eye syndrome with Lycium barbarum glycopeptide, and achieve the effect of safe consumption and significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
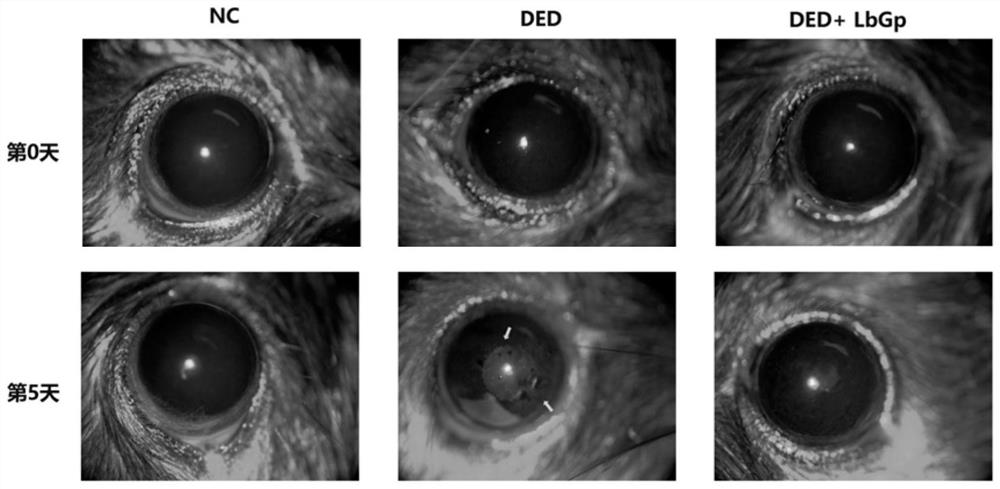
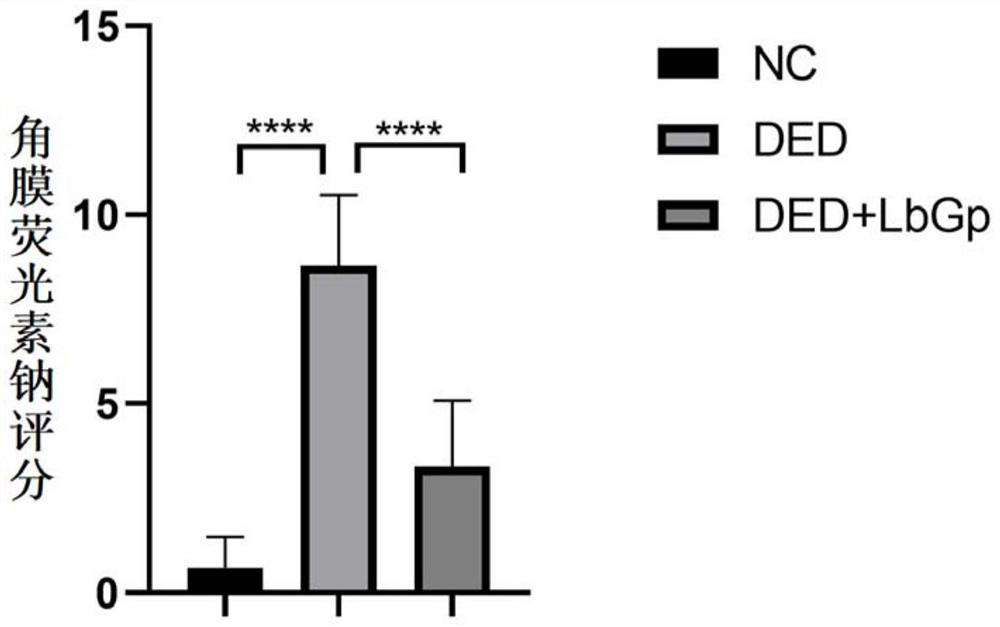
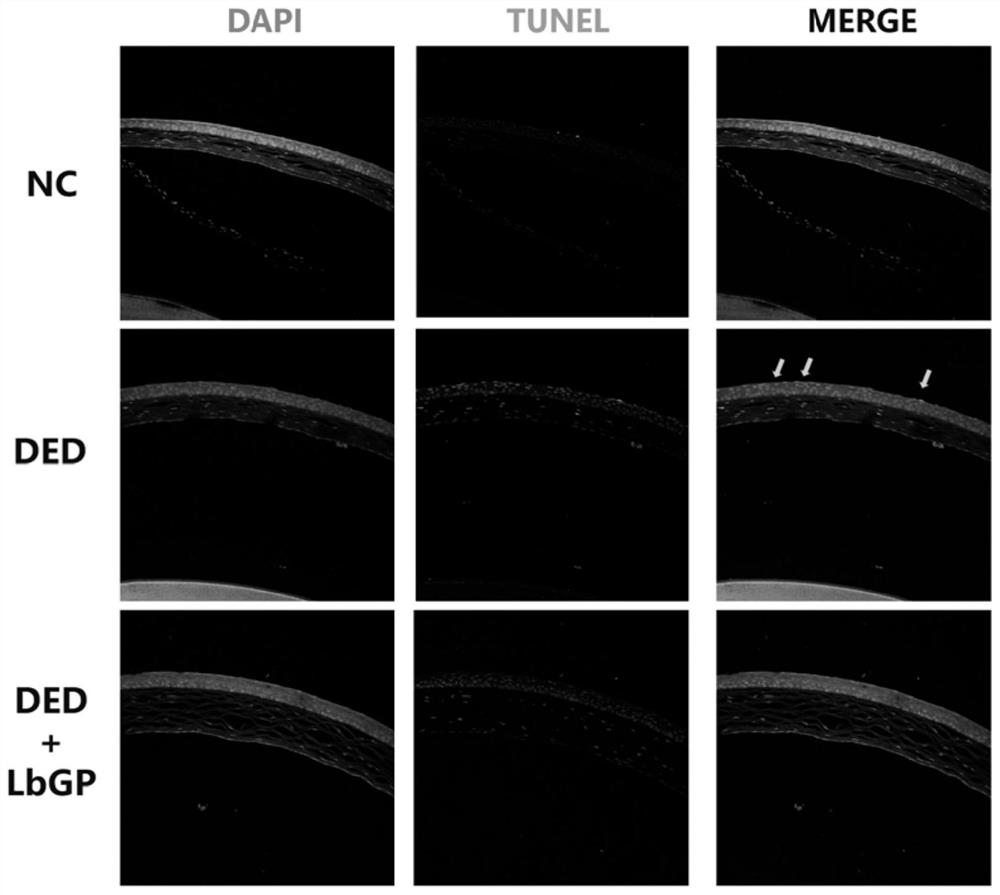
[0049] Example: Investigate the therapeutic effect of Lycium barbarum glycopeptide on dry eye model mice
[0050] 1. Experimental materials
[0051] 1.1 Experimental animals
[0052] This experiment selected SPF grade C57 / BL6 mice, female, 6 weeks old, body weight 20±2g, provided by Beijing Huafukang Biotechnology Co., Ltd. (license number: SCXK (Beijing) 2020-0004). Formal review and approval by the Animal Care and Ethics Committee of Zhongshan Eye Center (ethics batch number: 2021-013).
[0053] 1.2 Test reagents and their sources
[0054] Choline hydrobromide powder was purchased from Sigma Company in the United States, and its product number was R427039-250mg; fluorescein sodium ophthalmic test strip was purchased from Tianjin Jingming New Technology Development Co., Ltd., whose production batch number was 20211224; one-step TUNEL cell apoptosis The detection kit was purchased from Shanghai Beyotime Biotechnology Co., Ltd., and its product number was BWN-Beyotime-C1088....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com