Preparation method of N-cyclopropyl methylaniline compound
A technology of cyclopropylmethylaniline and compounds, which is applied in the field of preparation of N-cyclopropylmethylaniline compounds, can solve the problems of a large amount of solid residue and post-processing difficulties, achieve less impurities, reduce post-processing operations, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
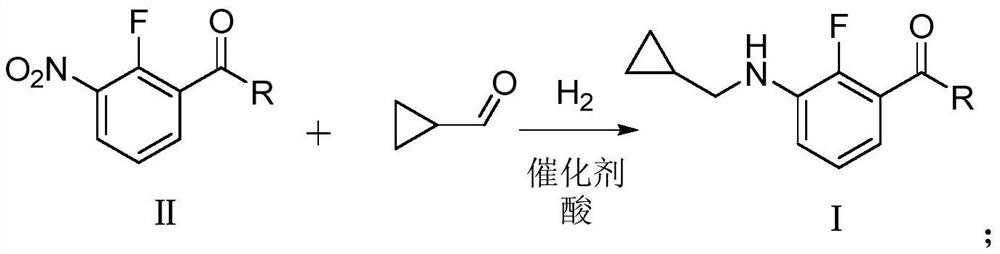
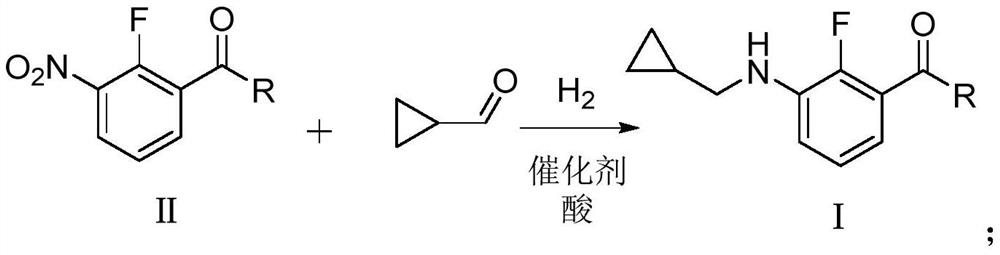
[0059] The present embodiment prepares 3-[(cyclopropylmethyl)amino]-2-fluorobenzoic acid methyl ester, and the reaction formula is as follows:
[0060]
[0061] Into the 500mL autoclave, 40.2g of 3-nitro-2-fluoro-methyl benzoate (0.2mol, 99% purity), 0.2g of 5% platinum carbon catalyst, 7.96g of acetic acid (0.13mol, 99% purity), Cyclopropylcarbaldehyde 16.8g (0.24mol, purity 99%) and methanol 119.4g were hydrogenated to a pressure of 1.0MPa and reacted at 40°C for 12h. After the reaction, filter, wash the filter residue with 20g methanol, combine the filtrates to remove the solvent under reduced pressure, and dry to obtain 43.2g of methyl 3-[(cyclopropylmethyl)amino]-2-fluorobenzoate, content 98.5% (external standard method, the same below), the yield was 95.4% (mass yield, the same below).
[0062] The characterization data is as follows:
[0063] LC / MS [M+1]: m / z=224.
[0064] 1 H NMR (400MHz, CDCl3) data as follows (δ[ppm]): 7.18-7.15 (m, 1H), 7.05-7.01 (m, 1H), 6.8...
Embodiment 2
[0066] The present embodiment prepares 3-[(cyclopropylmethyl)amino]-2-fluorobenzoic acid ethyl ester, and the reaction formula is as follows:
[0067]
[0068] In the 500mL autoclave, 43.0g of 3-nitro-2-fluoro-ethyl benzoate (0.2mol, 99% purity), 0.42g of 5% platinum carbon catalyst, 8.52g of acetic acid (0.14mol, 99% purity), Cyclopropylcarbaldehyde 19.6g (0.28mol, purity 99%) and ethanol 213g were hydrogenated to a pressure of 2.0MPa and reacted at 60°C for 14h. After the reaction, filter, wash the filter residue with 20g ethanol, combine the filtrates to remove the solvent under reduced pressure, and dry to obtain 3-[(cyclopropylmethyl)amino]-2-fluorobenzoic acid ethyl ester 45.1g, content 98.0%, yield was 93.2%.
[0069] Characterization data:
[0070] LC / MS [M+1]: m / z=238.
Embodiment 3
[0072] The present embodiment prepares 3-[(cyclopropylmethyl)amino]-2-fluorobenzoic acid propyl ester, and the reaction formula is as follows:
[0073]
[0074] In the 500mL autoclave, 45.9g of 3-nitro-2-fluoro-propyl benzoate (0.2mol, 99% purity), 0.91g of 5% platinum carbon catalyst, 18.16g of acetic acid (0.30mol, 99% purity), Cyclopropylcarbaldehyde 22.4 g (0.32 mol, purity 99%) and ethyl acetate 136.2 g were hydrogenated to a pressure of 3.0 MPa, and reacted at 100° C. for 16 h. After the reaction was completed, filter, wash the filter residue with 20 g of ethyl acetate, combine the filtrates to remove the solvent under reduced pressure, and dry to obtain 48.5 g of 3-[(cyclopropylmethyl)amino]-2-fluorobenzoic acid propyl ester with a content of 97.5%, The yield was 94.2%.
[0075] Characterization data:
[0076] LC / MS [M+1]: m / z=252.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com