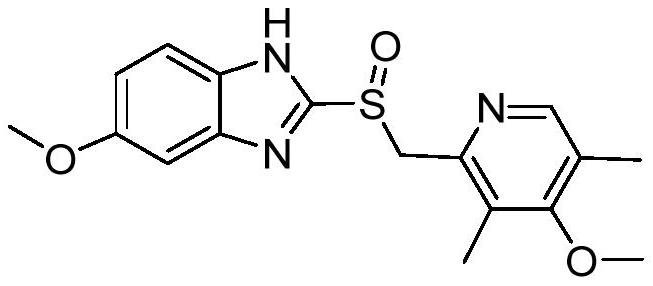
Preparation method of omeprazole intermediate
An omeprazole and intermediate technology, applied in the direction of organic chemistry, etc., can solve the problem of high total molar yield, and achieve the effects of high molar yield, high conversion rate and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
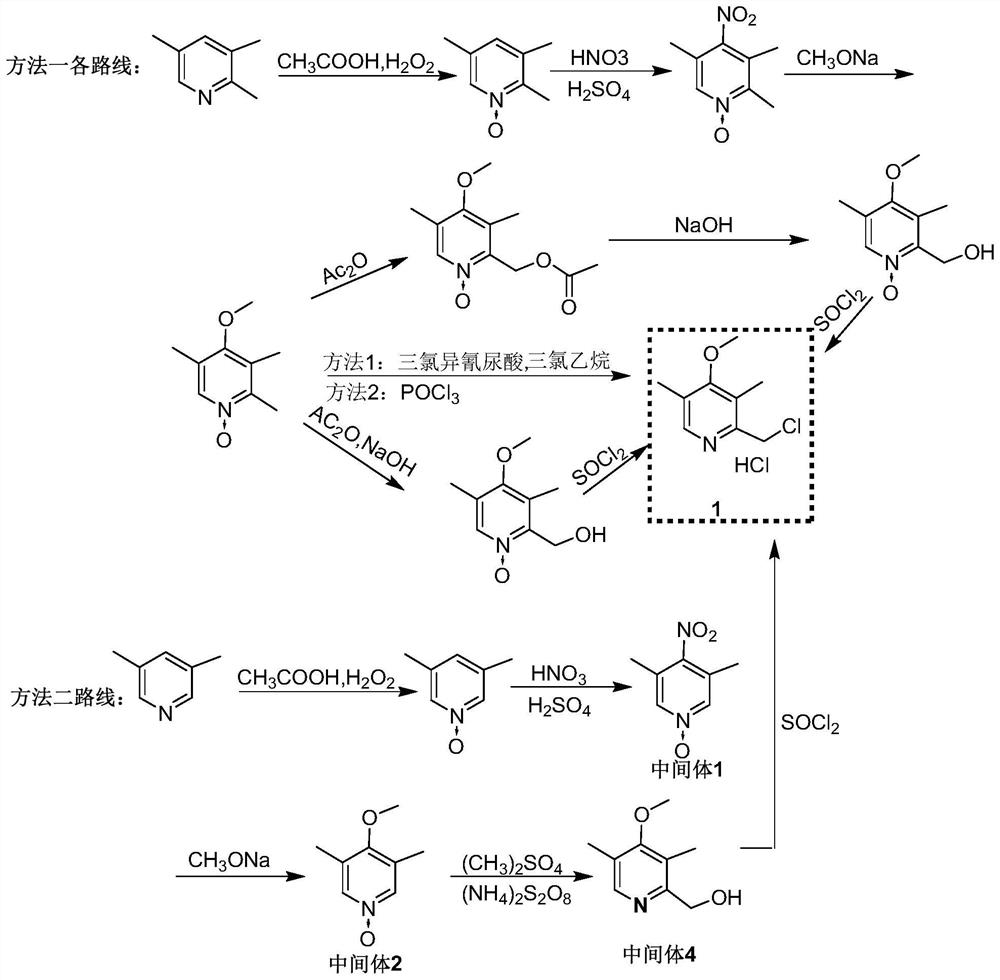
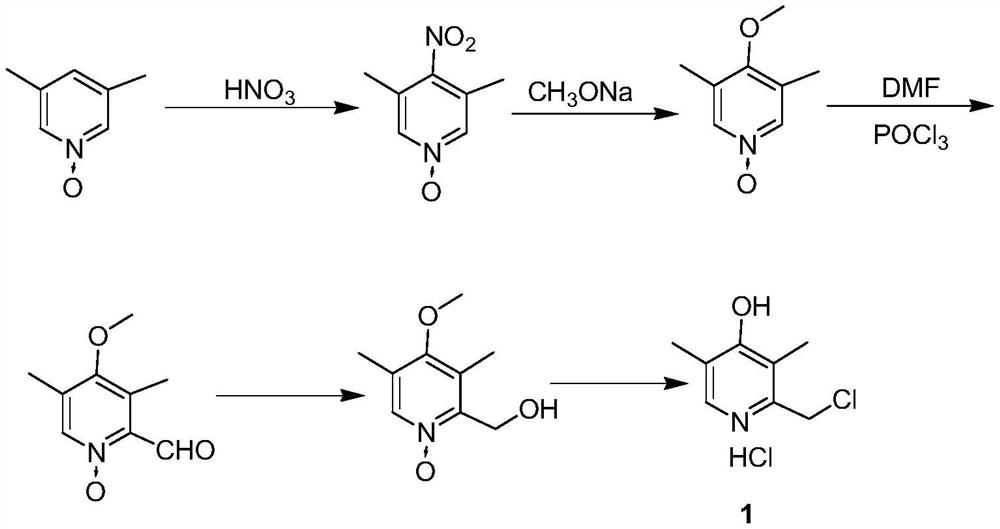
[0048] Preparation of 4-nitro-3,5-dimethyl-1-pyridine nitrogen oxide (intermediate 1)
[0049] Add 246.3g (2mol) 3,5-lutidine nitrogen oxide, 300g concentrated sulfuric acid (concentration 98%) to the reaction flask, heat up to 90 ℃, start to drip 385.7g (concentration 98%, 6mol) nitric acid and The mixed acid of 360g concentrated sulfuric acid (concentration 98%, 3.6mol) was added dropwise within 3.5h. After the dropwise addition was completed, the reaction was incubated and controlled by HPLC. In ice water, introduce ammonia gas under stirring to adjust pH to 2-3; control system temperature at 0 °C, continue stirring, filter, and collect filter cake; filter cake is dried under reduced pressure at 45 °C (vacuum degree≤-0.08MPa) to constant 323.9 g of 4-nitro-3,5-dimethyl-1-pyridine nitroxide (Intermediate 1) was obtained as a pale yellow solid, molar yield 96.3%, HPLC: 99.4%.
[0050] Preparation of 4-methoxy-3,5-lutidine-1-nitrogen oxide (intermediate 2)
[0051] 32.0g (0....
Embodiment 2
[0060] Preparation of Intermediate 1
[0061] Add 246.3g (2mol) 3,5-lutidine nitrogen oxide, 300g concentrated sulfuric acid (concentration 98%) to the reaction flask, heat up to 80°C, start dropwise addition of 385.7g (concentration 98%, 6mol) nitric acid and The mixed acid of 420g concentrated sulfuric acid (concentration 98%, 4.2mol) was added dropwise within 4h. After the dropwise addition was completed, the reaction was kept warm and controlled by HPLC. After the reaction was completed for 5h, the reaction of the raw materials was complete; In the water, pour liquid ammonia under stirring to adjust the pH to 2-3; control the temperature of the system at 0 °C, continue stirring, filter, and collect the filter cake; the filter cake is dried under reduced pressure at 45 °C (vacuum degree≤-0.08MPa) to constant weight , to obtain 330.2 g of 4-nitro-3,5-dimethyl-1-pyridine nitrogen oxide (Intermediate 1), a pale yellow solid, molar yield 98.2%, HPLC: 99.5%.
[0062] Preparatio...
Embodiment 3
[0072] Preparation of Intermediate 1
[0073] 246.3g (2mol) of 3,5-lutidine nitrogen oxide, 300g of concentrated sulfuric acid (98% concentration) were added to the reaction flask, the temperature of the system was increased to 110°C, and 385.7g (98% concentration, 6mol) of nitric acid and The mixed acid of 600g concentrated sulfuric acid (concentration 98%, 6mol) was added dropwise within 4.5h, the reaction was kept warm, controlled by HPLC, and the reaction of the raw materials was complete after 5h of reaction; the reaction solution was cooled to room temperature, added dropwise to 1000g of ice water, and stirred Introduce liquid ammonia, adjust the pH to 3-4; control the temperature of the system at 0 °C, continue stirring, filter, collect the filter cake, and dry the filter cake under reduced pressure at 45 °C (vacuum degree≤-0.08MPa) to constant weight to obtain 4- Nitro-3,5-dimethyl-1-pyridine nitrogen oxide (Intermediate 1) 327.6 g, pale yellow solid, molar yield 97.4%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com