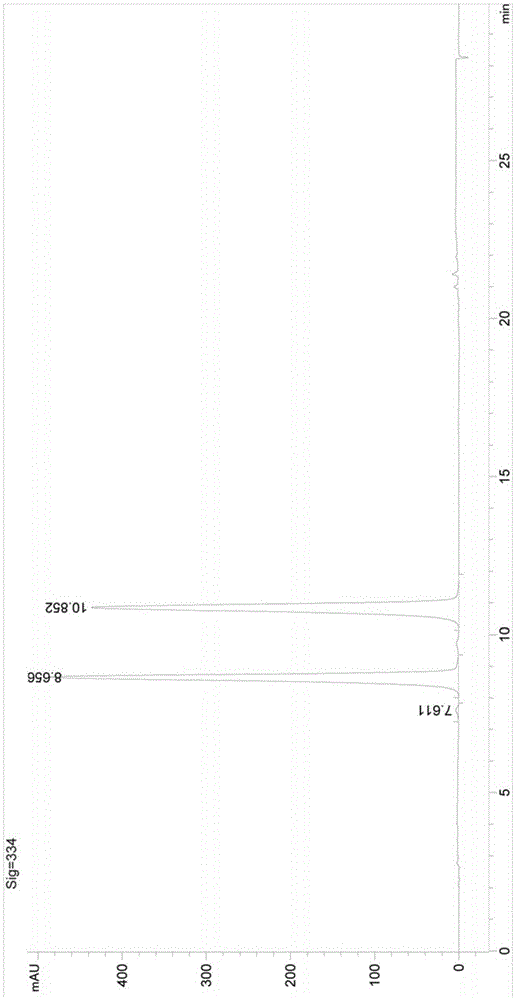
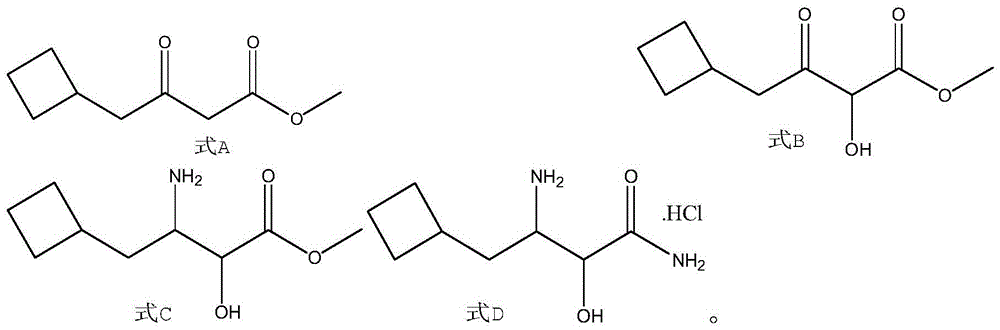
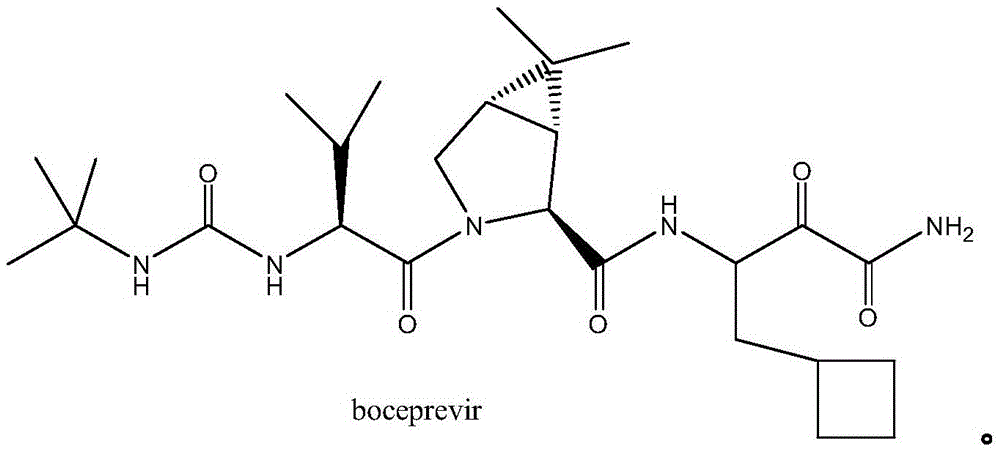
A kind of synthetic method of boceprevir intermediate 2-hydroxyl-3-amino-4-cyclobutylbutanamide hydrochloride
A technology of cyclobutylbutanamide and synthesis method, which is applied in the field of synthesis of boceprevir intermediates, can solve the problems of high equipment requirements, cumbersome handling, lengthy reaction steps, etc., and achieve simple post-treatment operation, simple reaction conditions, The effect of shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] At room temperature, 50 g (0.43 mol) of cyclobutylacetic acid, 73.4 g (0.47 mol) of monomethyl malonate potassium salt, and 300 mL of tetrahydrofuran were successively added to the three-necked reaction flask, and 103.7 g (0.64 mol) of carbonyldiimidazole was added under stirring. ), magnesium chloride 15g to form a reaction solution, after the addition, the reaction solution was reacted at room temperature for 12h, then 100mL of water was added to the reaction solution, stirring was continued for 0.5h, liquid separation, the organic phase continued to be washed once with saturated brine 50mL, and dried. Concentration gave 66.5 g of the compound of formula A 4-cyclobutyl-3-oxo-butyric acid methyl ester, with a yield of 89.2%.
[0039] Add 200mL of formic acid to the three-necked reaction flask, add sodium percarbonate 33.2g (0.2mol), 4-cyclobutyl-3-oxo-butyric acid methyl ester 66.5g (0.39mol), iodine 4 g (0.02 mol) of benzene forms a reaction solution. After the additi...
Embodiment 2
[0043] At room temperature, 30 g (0.26 mol) of cyclobutylacetic acid, 44 g (0.28 mol) of monomethyl malonate potassium salt, and 300 mL of 2-methyltetrahydrofuran were successively added to the three-necked reaction flask, and after stirring, 47.8 cyanuric chloride was added. g (0.26mol), magnesium chloride 10g to form a reaction solution, after the addition, react the reaction solution at room temperature for 10h, then add 100mL of water to the reaction solution, continue to stir for 0.4h, separate the liquids, and continue to wash the organic phase with 50mL of saturated saline Once, dried and concentrated to obtain 39.5 g of the compound of formula A, 4-cyclobutyl-3-oxo-butyric acid methyl ester, with a yield of 88.5%.
[0044] Add 150mL of formic acid to the three-necked reaction flask, add sodium perborate 35.8g (0.23mol), 4-cyclobutyl-3-oxo-butyric acid methyl ester 39.5g (0.23mol), iodine 3 g (0.02 mol) of benzene forms a reaction solution, and after the addition, keep ...
Embodiment 3
[0048] At room temperature, add 18 g (0.16 mol) of cyclobutylacetic acid, 24.6 g (0.16 mol) of monomethyl malonate potassium salt, and 200 mL of ethylene glycol dimethyl ether into the three-necked reaction flask in sequence, and continue to add dicarbonate dicarbonate after stirring. 35.04g (0.16mol) of tert-butyl ester and 10g of magnesium chloride form a reaction solution. After the addition, the reaction solution is reacted at room temperature for 11h, then 100mL of water is added to the reaction solution, and the stirring is continued for 0.6h. Wash once with 50 mL of saline, dry, and concentrate to obtain 22.3 g of the compound of formula A, 4-cyclobutyl-3-oxo-butyric acid methyl ester, with a yield of 83.1%.
[0049] Add 150mL of formic acid to the three-necked reaction flask, then add 4.4g (0.13mol) of hydrogen peroxide, 13.78g (0.13mol) of sodium carbonate, and 22g of methyl 4-cyclobutyl-3-oxo-butyrate at 15-20°C (0.13mol), iodobenzene 2g (0.01mol) to form a reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com