Triazine ring-containing polymer and film-forming composition containing same
A triazine ring and polymer technology, which is applied in the field of film-forming compositions, can solve the problems of not being able to use high-polarity solvents, and achieve the effects of improving light extraction efficiency, low volume shrinkage, and high transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
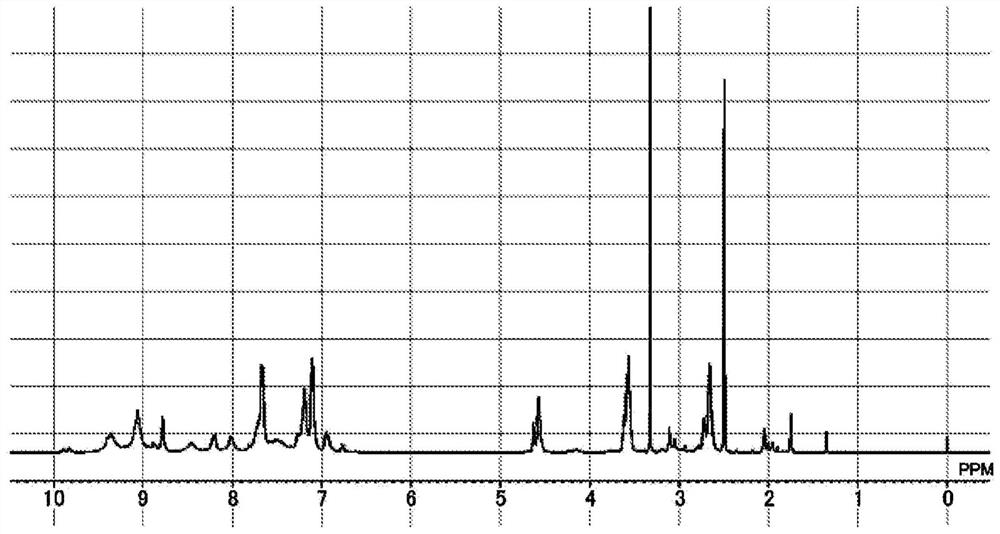
[0374] [Example 1-1] Synthesis of polymer compound [4]
[0375]
[0376]To a 1000 mL four-neck flask were added 1,3-phenylenediamine[2] (35.18 g, 0.325 mol, manufactured by Amino-Chem) and 666.3 g of dimethylacetamide (DMAc, Kanto Chemical). Co., Ltd.), after nitrogen substitution, stirring was performed so that 1, 3- phenylenediamine [2] was dissolved in DMAc. After that, it was cooled to -10°C in an ethanol-dry ice bath, and 2,4,6-trichloro-1,3,5-triazine[1] (60.0 g, 0.325 mol) was added while checking that the internal temperature did not become 0°C or higher. , manufactured by Tokyo Chemical Industry Co., Ltd.). After stirring for 30 minutes, the oil bath was set to 90 to 100°C, and the temperature of the reaction solution was raised to an internal temperature of 85°C±5°C. After stirring at an internal temperature of 85°C for 1 hour, 2-(4-aminophenyl)ethanol [3] (53.56 g, 0.456 mol, manufactured by Oakwood Corporation) was preliminarily dissolved in 107.12 g of DMAc, ...
Embodiment 1-2
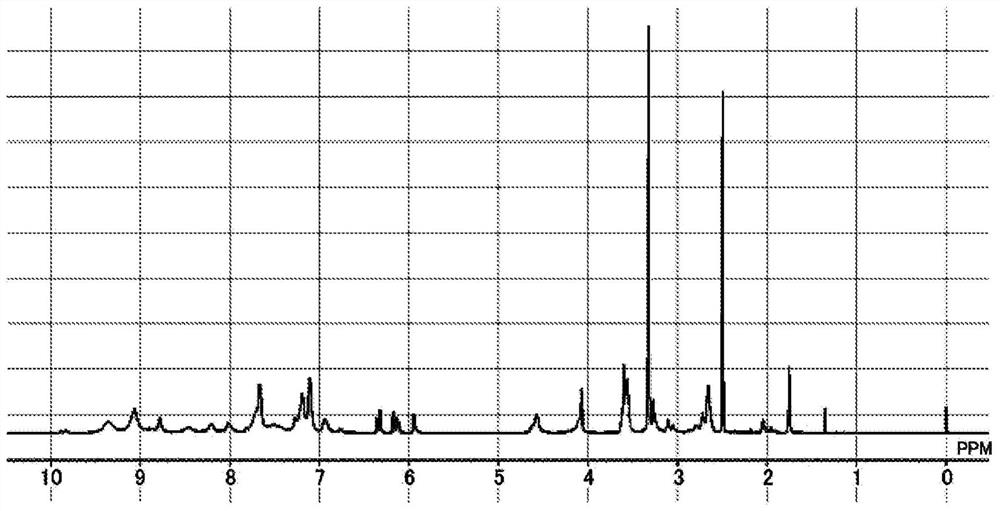
[0378] [Example 1-2] Synthesis of polymer compound [5]
[0379]
[0380] To a 150 mL four-neck flask, 25.0 g of compound P-1 synthesized in Example 1-1, 100.0 g of THF (manufactured by Junsei Chemical Co., Ltd.), and 11.66 g of pure water were added, and after nitrogen substitution, stirring was performed to dissolve P-1. in THF. Then, it heated up to 60 degreeC of internal temperature, 12.70g of 2-isocyanatoethyl acrylates (Karenz AOI, the Showa Denko Co., Ltd. make) were added, and it stirred for 3 hours. Thereafter, the temperature was lowered to room temperature, and the reaction solution was added dropwise to a mixed solution of methanol (149 g) and ion-exchanged water (448 g) to reprecipitate. The obtained precipitate was separated by filtration and dried at 80° C. for 3 hours using a vacuum dryer to obtain 21.6 g of the target polymer compound [5] (hereinafter, referred to as P-2). The compound P-2 1 The measurement results of the H-NMR spectrum are shown in fig...
Embodiment 1-3
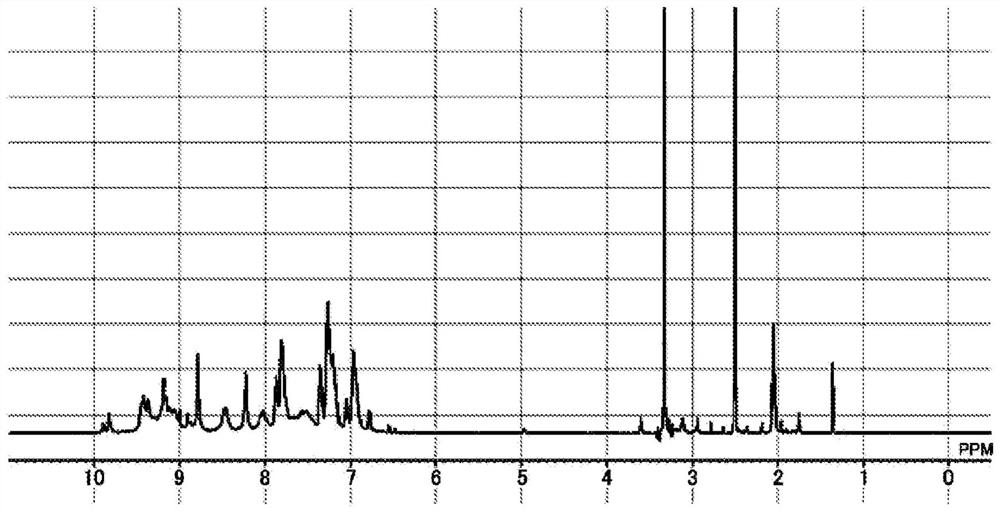
[0382] [Example 1-3] Synthesis of polymer compound [7]
[0383]
[0384] To a 500 mL four-necked flask were added 1,3-phenylenediamine[2] (11.73 g, 0.108 mol, manufactured by Arno Chemicals) and 204.96 g of dimethylacetamide (DMAc, manufactured by Kanto Chemical Co., Ltd.), After nitrogen replacement, stirring was performed to dissolve 1,3-phenylenediamine [2] in DMAc. After that, it was cooled to -10°C in an ethanol-dry ice bath, and 2,4,6-trichloro-1,3,5-triazine[1] (20.00 g, 0.108 mol) was added while checking that the internal temperature did not become 0°C or higher. , manufactured by Tokyo Chemical Industry Co., Ltd.). After stirring for 30 minutes, 87.8 g of DMAc was preliminarily added to a 500 mL four-necked flask, and after nitrogen replacement, the oil bath was set to 90 to 100°C so that the inner temperature was 85°C±5°C, and the reaction solution was added dropwise. After stirring at an internal temperature of 85°C for 1 hour, aniline [6] (10.10 g, 0.108 mol,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polydispersity | aaaaa | aaaaa |
| Polydispersity | aaaaa | aaaaa |
| Polydispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com