Method for converting n-alkane into fully conjugated polyene
A technology of n-alkanes and conjugated polyenes, applied in chemical instruments and methods, preparation of organic compounds, catalysts, etc., can solve problems such as unreported synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
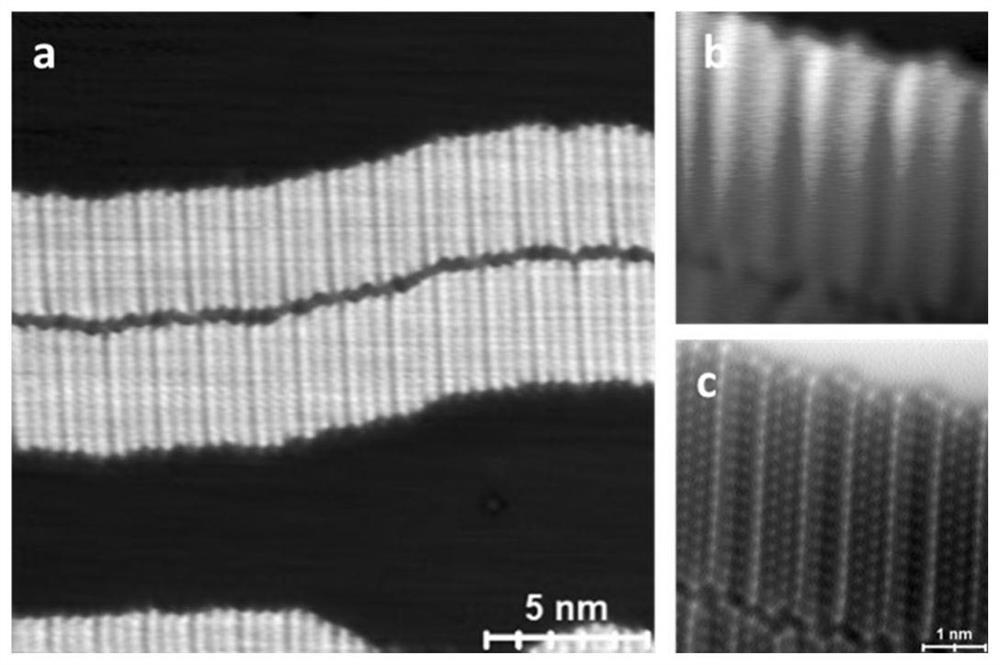
[0037] Example 1: n-docosane (molecule (I)) was grown on the surface of Cu(110) single crystal cleaned by argon sputtering annealing by organic molecular beam epitaxy, wherein the diameter of the substrate was 9 mm, and the lining was The bottom temperature is room temperature, and the deposition environment is ultra-high vacuum (1x10 -10 mbar), the deposition temperature was 100 degrees Celsius, and the deposition time was 5 minutes. The deposited samples were characterized by scanning tunneling microscopy (Omicron LT-STM, Germany) as follows figure 1 a shown. Due to the mismatch between the molecular and the substrate period in the assembled structure, in scanning tunneling microscopy, such as figure 1 A superstructure pattern with a period of 14.7 Å is shown in b. The frequency-shifted images obtained by in situ scanning with a non-intrusive atomic force microscope (Omicron LT-STM, Germany) are as follows: figure 1 c shown.
[0038] The samples were annealed at 180°C f...
Embodiment 2
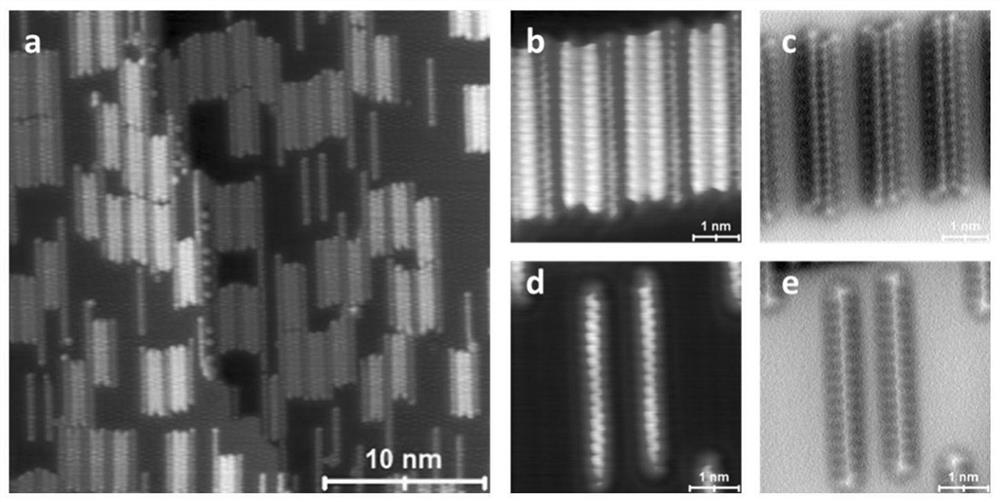
[0039] Example 2: growing n-docosane (molecule (I)) onto a clean Cu (110) single crystal surface by organic molecular beam epitaxy, wherein the substrate diameter is 9 mm, the substrate temperature is room temperature, and the deposition The environment is ultra-high vacuum (1x10 -10 mbar), the deposition temperature was 105 degrees Celsius, and the deposition time was 10 minutes. The deposited samples were characterized by scanning tunneling microscopy as Figure 4 a shown.
[0040] The alkane-deposited sample was heated by a thermal radiation heating stage at a constant heating power (5 watts) for one hour, and the substrate temperature measured by the thermocouple was 196.2 degrees Celsius. The heat-treated samples were characterized by scanning tunneling microscopy, and the results were as follows Figure 4 b shown. There are both unreacted alkane molecules and converted olefin molecules on the surface.
[0041] The samples were further annealed at higher heating powe...
Embodiment 3
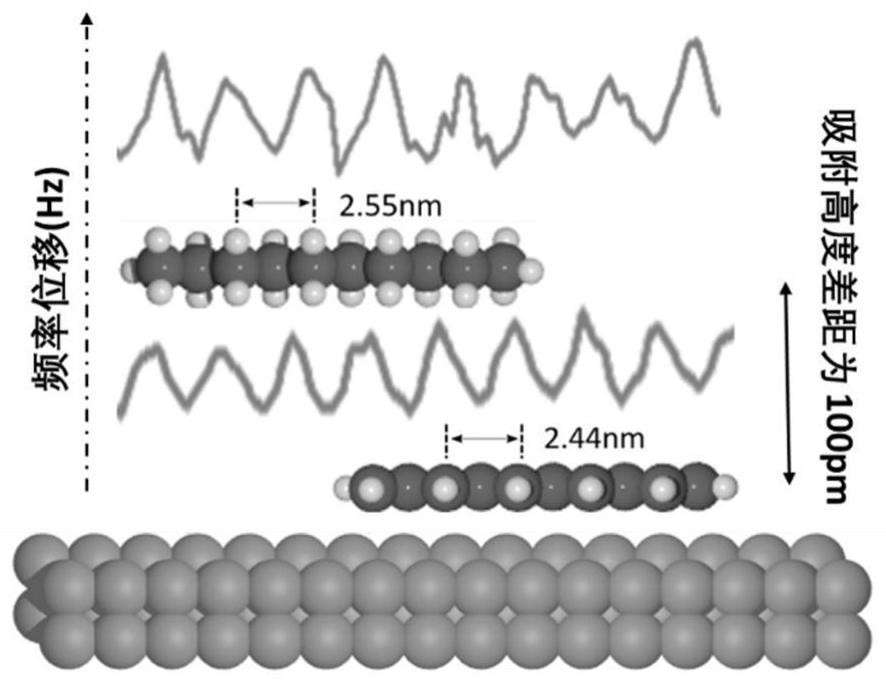
[0043] Example 3: Octadecylbenzene (Molecule (III)) was grown on a clean Cu(110) single crystal surface by organic molecular beam epitaxy, wherein the substrate diameter was 9 mm, the substrate temperature was room temperature, and the deposition environment for ultra-high vacuum (1x10 -10 mbar), the deposition temperature was 40 degrees Celsius, and the deposition time was 2 minutes. The deposited samples were characterized by scanning tunneling microscopy as Figure 5 a shown.
[0044] The samples were annealed at 120°C for one hour in an ultra-high vacuum chamber using a thermal radiation heating stage. The treated samples were characterized by scanning tunneling microscopy as Figure 5 b shown. The originally assembled octadecylbenzene molecules became discrete monomers, implying that the conversion of alkanes to polyenes took place. Characterized by non-contact atomic force microscopy, the alkane components in the dispersed single molecules have all been converted in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com