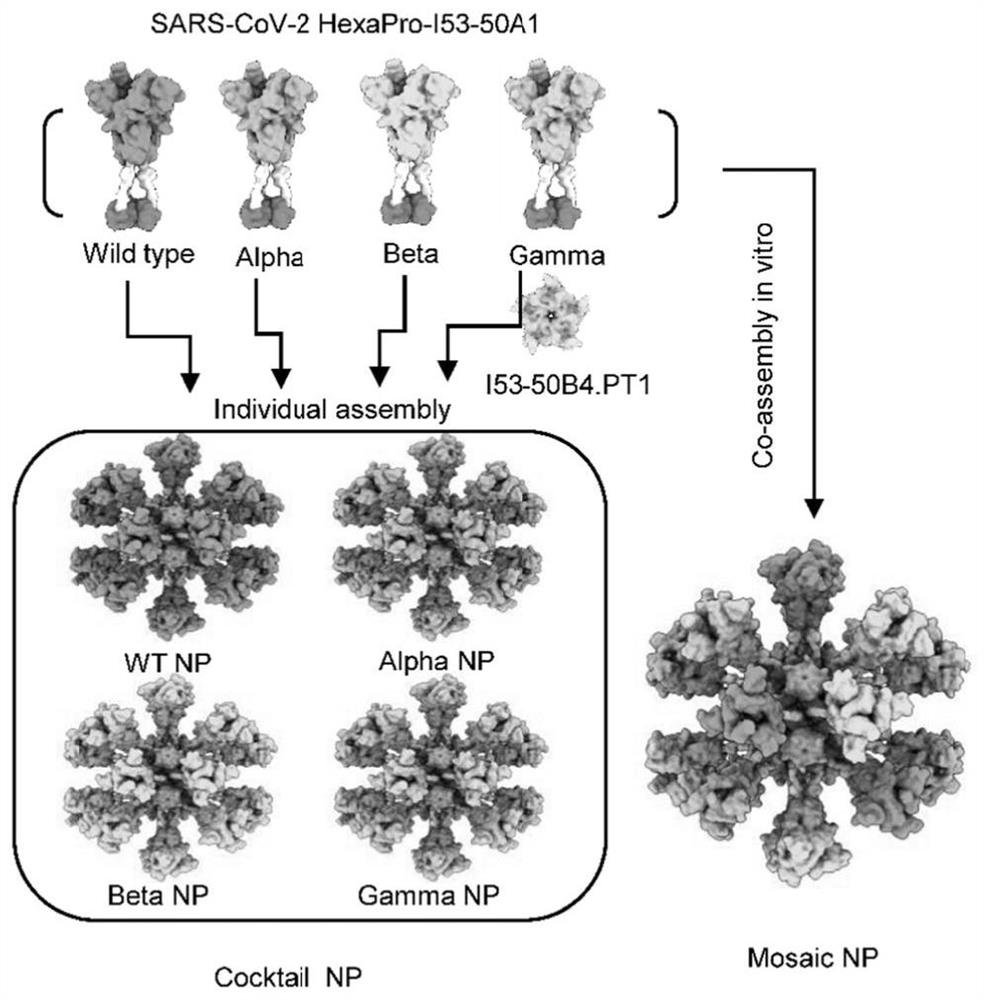
Quadrivalent SARS-CoV-2 chimeric nanoparticle vaccine as well as preparation method and application thereof
A nanoparticle and vaccine technology, applied in the field of biology, can solve the problems of serum immune escape, affect antigenicity, and low yield, and achieve the effects of improving immunogenicity, enhancing humoral immune response, and increasing expression yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The preparation method of the tetravalent chimeric nanoparticles of the present application comprises the following steps:
[0067] 1. Through computer-aided design such as Sic_axle and Rosetta, the fusion distance between the antigen and the nanoparticle carrier was determined, and the natural trimer domain of T4 phage fibrin was introduced at the same time, so as to determine the hinge for nanoparticle design in the sequence (linker) Length and antigen trimerization and selection of nanoparticle carriers based on the length design.
[0068] 2. Through the first host cell, different eukaryotic expression vectors were transferred into the first cell by transient transfection technology, respectively, to obtain different nanoparticle subunits of HexaPro-I53-50A1 (the first nanoparticle subunit to The fourth nanoparticle subunit), while using the second host cell, transform another I53-50B.4PT1 expression plasmid, and express after IPTG induction to obtain another nanopar...
Embodiment 1
[0071] Example 1 Construction, Expression and Purification of Chimeric Quadrivalent SARS-CoV-2 HexaPro Nanoparticle Vaccine
[0072] 1.1 Design of SARS-CoV-2 bicomponent antigen
[0073] Through computer-aided design such as Sic_axle and Rosetta, the HexaPro antigen (SARS-CoV-2) of SARS-CoV-2 wild type and VOCs (British strain, South African strain and Brazilian strain, corresponding to Alpha, Beta and Gamma types, respectively) was determined. The amino acid sequences of the wild-type, Alpha-type, Beta-type and Gamma-type HexaPro antigens are SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4 in sequence) and the nanoparticle carrier (I53- 50A1) fusion gap; between the HexaPro antigen and the trimerized I53-50A1 subunit (SEQ ID NO: 5), the native trimer domain (SEQ ID NO: 6) of T4 phage fibrin was introduced, a segment comprising 16 A flexible linker of glycine-serine residues (SEQ ID NO: 7) and a rigid linker (SEQ ID NO: 8); for subsequent purification of the trimeri...
Embodiment 2
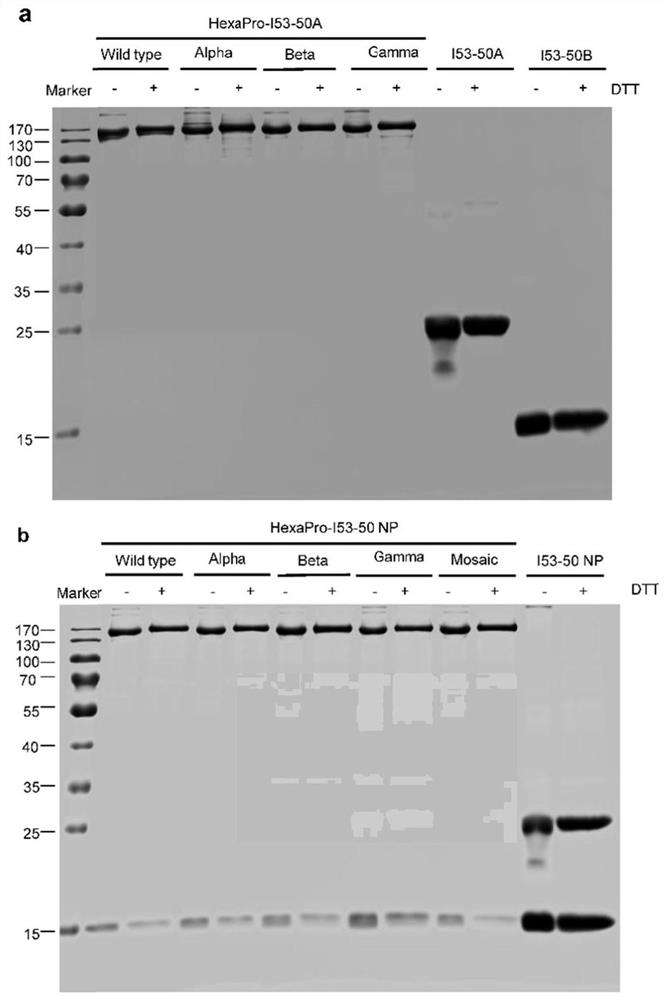
[0085] Example 2 Biophysical properties of chimeric tetravalent nanoparticle immunogens
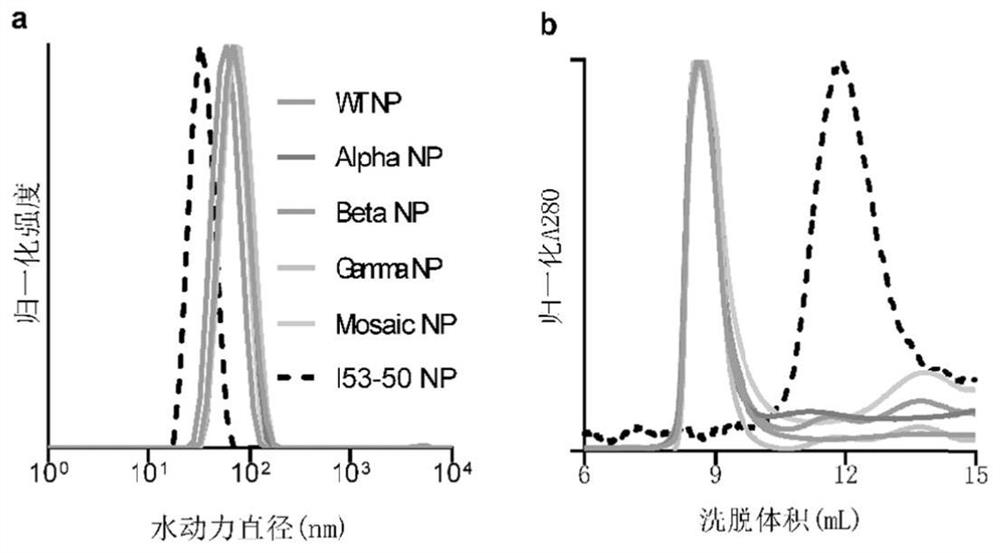
[0086] 2.1 Particle size and distribution of nanoparticle immunogens
[0087] The four SARS-CoV-2 HexaPro-I53-50 NPs, Mosaic NPs and empty particles (I53-50 NPs) obtained in Example 1 were diluted with PBS solution to a concentration of 0.5 mg / mL; 50 μL were diluted The sample was added to a disposable solvent-resistant micro-test tube, and allowed to stand at 25°C for 2 min. The particle size of the nanoparticles was detected using a Zetasizer Ultra dynamic light scattering instrument (Malvern Panalytical), and the measurement angle was set to 173°, which was determined by measuring the intensity of scattered light. The size distribution of the protein was measured 5 times for each sample, and the average value was obtained to obtain the particle size.
[0088] like image 3 As shown in a, each nanoparticle immunogen is unimodal, the distribution maps of the four SARS-CoV-2 HexaPro-I53...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com