Pig viral diarrhea detection primer combination, detection kit and application
A swine viral diarrhea, detection kit technology, applied in the directions of microorganisms, recombinant DNA technology, microorganism-based methods, etc., can solve the problems of multi-virus mixed infection, unable to meet the needs of epidemic disease detection, and the lack of mature detection methods for viruses, etc. To achieve the effect of convenient detection, high accuracy and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] This embodiment provides a swine viral diarrhea detection kit, which includes a positive control, a negative control, a primer premix, a probe premix, a PCR amplification solution, and an enzyme premix.
[0100] Positive control: recombinant plasmids containing PEDV, TGEV, RV, PDCoV target gene fragments respectively.
[0101] Negative control: double distilled water.
[0102] Primer master mix: including PEDV-F, PEDV-R, TGEV-F, TGEV-R, RV-F, RV-R, PDCoV-F, PDCoV-R at an initial concentration of 10 μM in a molar ratio of 1:1:1: 1:1:1:1:1 premix;
[0103] Probe premix: PEDV-P, TGEV-P, RV-P, PDCoV-P with an initial concentration of 10 μM in a molar ratio of 1:1:1:1;
[0104] PCR amplification solution includes: 2×One Step U+ (Novizan);
[0105] Enzyme premix: One Step U+Enzyme Mix (Novizan).
[0106] Upstream primer PEDV-F (SEQ ID NO.1):
[0107] 5'-CCAACTGGTGTAACGCTAAC-3'
[0108] Downstream primer PEDV-R (SEQ ID NO. 2):
[0109] 5'-GACATAGAAAGCCCAACCAG-3'
[011...
experiment example 1
[0142] In this experiment, the conditions of quadruple fluorescence quantitative PCR were optimized.
[0143] Use Tiangen's plasmid extraction kit to extract the plasmids of the positive strains with correct sequencing in Example 1, use the NanoDrop 2000 nucleic acid concentration analyzer to detect the concentration of the plasmid template, and dilute the four standard plasmids by 10-fold gradient to take the concentration of 10 4copise / μL, 1:1:1:1 was mixed as template. The total system is 30 μL, the upstream and downstream primers and corresponding probes are mixed with different final probe concentrations and primer final concentrations, respectively, and 1.2 μL and 1.2 μL of primers (upstream and downstream) and corresponding probes (the total upstream and downstream primer concentrations) are added respectively. 400nM: probe concentration 400nM); 1.2μL, 0.6μL (upstream and downstream total primer concentration 400nM: probe concentration 200nM), 1.2μL, 0.3μL (upstream and...
experiment example 2
[0152] This experimental example is a repeatable verification experiment.
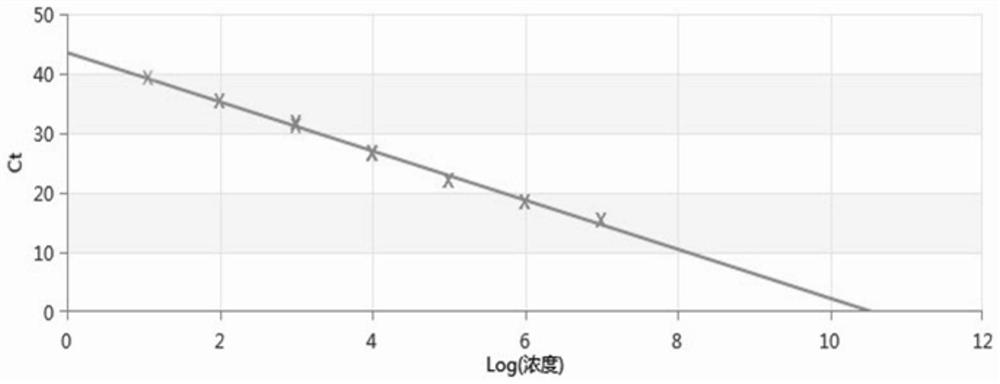
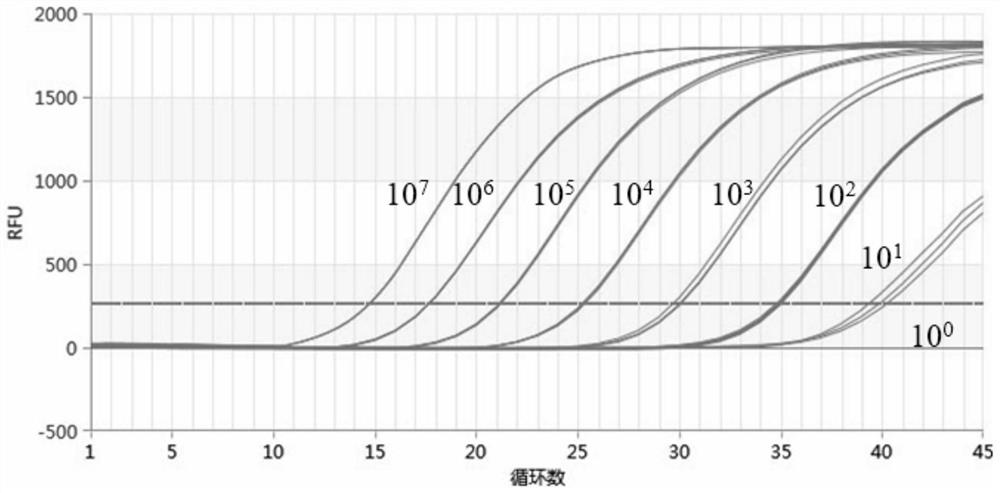
[0153] The positive control plasmids of 7 concentrations of 10-fold serial dilution were used as templates, and the final concentrations were 10 7 , 10 6 , 10 5 , 10 4 , 10 3 , 10 2 , 10 1 , 10 0 copise / μL, and perform fluorescence quantitative PCR according to the reaction system and program that provides fluorescence quantification, and set 3 replicates for each gradient to verify the repeatability of the method. The results show that the coefficient of variation (CV value) of the repeated experiments of the present invention is all below 0.5%, indicating that the detection kit provided by the present invention has good repeatability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com