In-situ gelling chemotherapy immune medicine composition and preparation method thereof
A chemoimmune and composition technology, applied in the field of tumor treatment drugs, can solve problems such as single administration mode, limited application, etc., and achieve the effects of ensuring water dispersibility and stability, avoiding pH changes, and improving stability problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
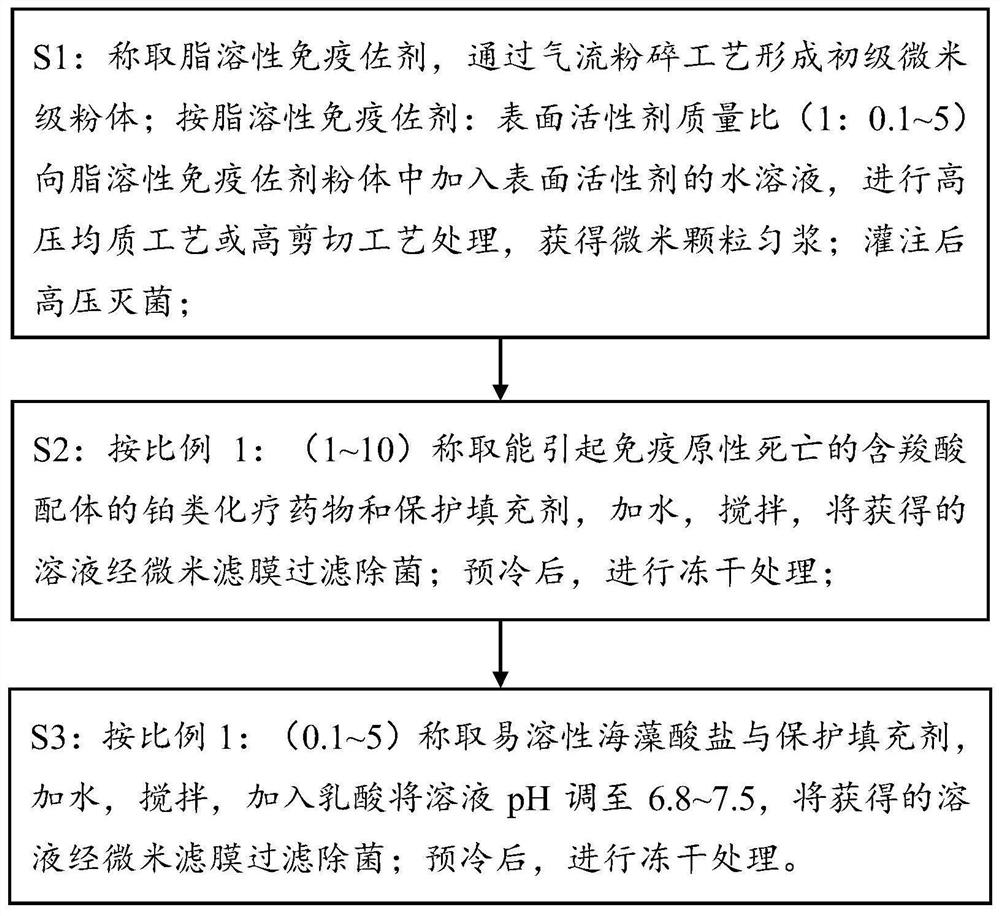
[0046] The preparation method of the first composition:
[0047] S1: Weigh a certain amount of the solid of the fat-soluble immune adjuvant imiquimod R837 and carry out jet pulverization treatment, and the pulverization pressure is 6-10 bar to obtain micron-scale imiquimod R837. Proportion 1: (0.025~5) Weigh micron-sized fat-soluble immune adjuvant imiquimod R837 and surfactant poloxamer 188, preferably 2g R837, add an appropriate amount of poloxamer 188 (0.05g, 0.3g g, 0.6g, 1g, 2g, 4g, 6g, 8g, 10g), add 200ml of water for injection, and stir at 100-500rpm for 0.5-2 hours to obtain a suspension. Homogenize the above suspension under high pressure for 2-4 times under the pressure of 750-1200bar, suck the suspension with a peristaltic pump and fill it into 10ml ampoules, each bottle is 6ml, for a total of 30 bottles. After melting and sealing, a micron suspension is obtained, which is sterilized by moist heat at 105°C to 150°C for 15-20 minutes.
[0048] The preparation metho...
Embodiment A2
[0058] The preparation method of the first composition:
[0059] S1: Weigh a certain amount of the fat-soluble immune adjuvant Resiquimod R848 solid and carry out jet pulverization treatment, and the pulverization pressure is 6-10 bar to obtain micron-scale Resiquimod R848. Weigh micron-sized fat-soluble immune adjuvant Resiquimod R848 and surfactant poloxamer 407, preferably 0.2g R848, according to the ratio 1:(0.025~5), add an appropriate amount of poloxamer 407 (0.005g, 0.05g, 0.1g, 0.2g, 0.4g, 0.8g, 1g), add 200ml of water for injection, and stir at 100-500rpm for 0.5-2 hours to obtain a suspension. Homogenize the above suspension under high pressure for 2-4 times under the pressure of 750-1200bar, suck the suspension with a peristaltic pump and fill it into 10ml ampoules, each bottle is 6ml, for a total of 30 bottles. After melting and sealing, a micron suspension is obtained, which is sterilized by moist heat at 105°C to 150°C for 15-20 minutes.
[0060] The preparatio...
Embodiment B
[0069] Instructions for the use of the three-component mixed liquid and lyophilized preparations.
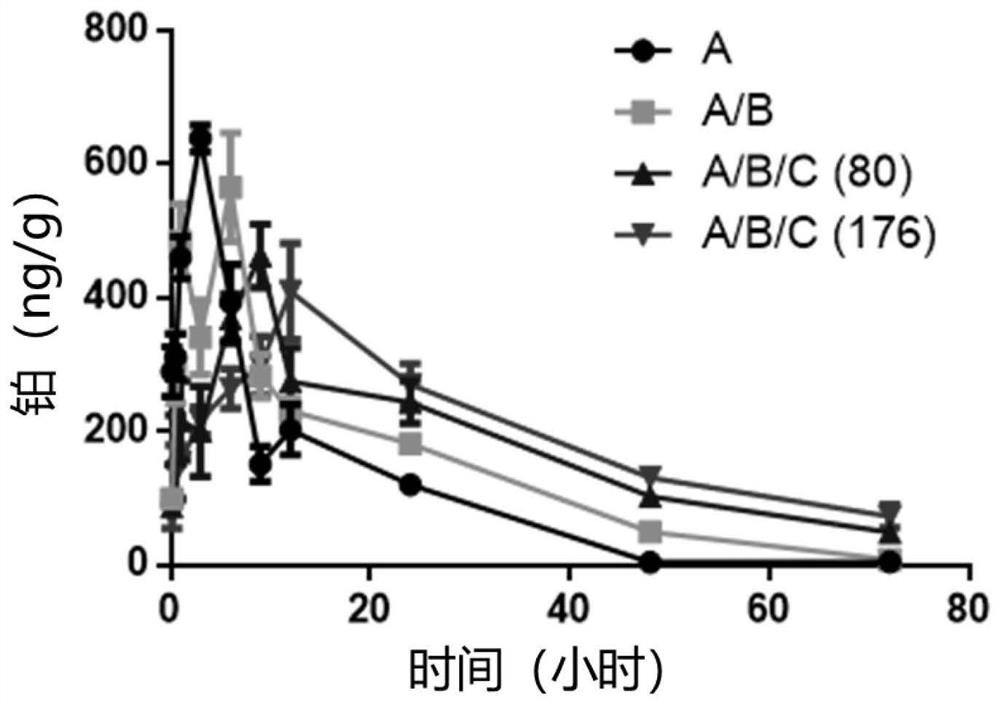
[0070] Use scheme 1: The freeze-dried powder injection of the composition of the second and third types of components in the above example A1 is added to the suspension of the first component composition and mixed evenly, and the drug is administered by clinical intervention and direct puncture. In the method, the composition solution is directly injected into the tumor site of the patient, and multi-point injection is adopted during injection to ensure that the composition solution evenly fills the entire tumor.
[0071] After the composition is injected into the tumor, firstly, when the alginate in the third composition encounters calcium ions in the organism tissue or the gel-forming excipients in the fourth composition, it will rapidly gel to form a porous network The combined structure enables the other three types of components mixed in alginate to be released slowly, ther...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com