Bimetal organic framework heterogeneously catalyzed Kumada coupling reaction
An organic framework and heterogeneous catalysis technology, applied in organic chemistry, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve expensive, difficult separation and recovery, and difficult to remove metal residues and other problems, to achieve the effect of reducing metal residues, large-scale catalytic application, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Preparation of bimetallic organic frameworks (MOFs): Different kinds of metal nitrates (0.6 mmol) and organic ligands were placed in N,N-dimethylformamide (2 mL), ethanol (2 mL) and water (2 mL) The mixed solution was stirred at room temperature to fully dissolve it, and then placed in a 120 °C oven for two days to react, cooled to room temperature, washed with ethanol and dried to finally obtain a bimetallic organic framework material.
[0055] In the above-mentioned preparation method: nitrate is cobalt nitrate hexahydrate, iron nitrate nonahydrate (III) or manganese nitrate tetrahydrate;
[0056] In the above preparation method: the molar ratio of different kinds of nitrates is about 1:1;
[0057]In the above-mentioned preparation method: the organic ligands are trimesic acid (BTC) and thiodiacetic acid (tda);
[0058] In the above preparation method: the molar ratio of nitrate, trimesic acid (BTC) and thiodiacetic acid (tda) is about 1:0.3:1;
Embodiment 1
[0061]
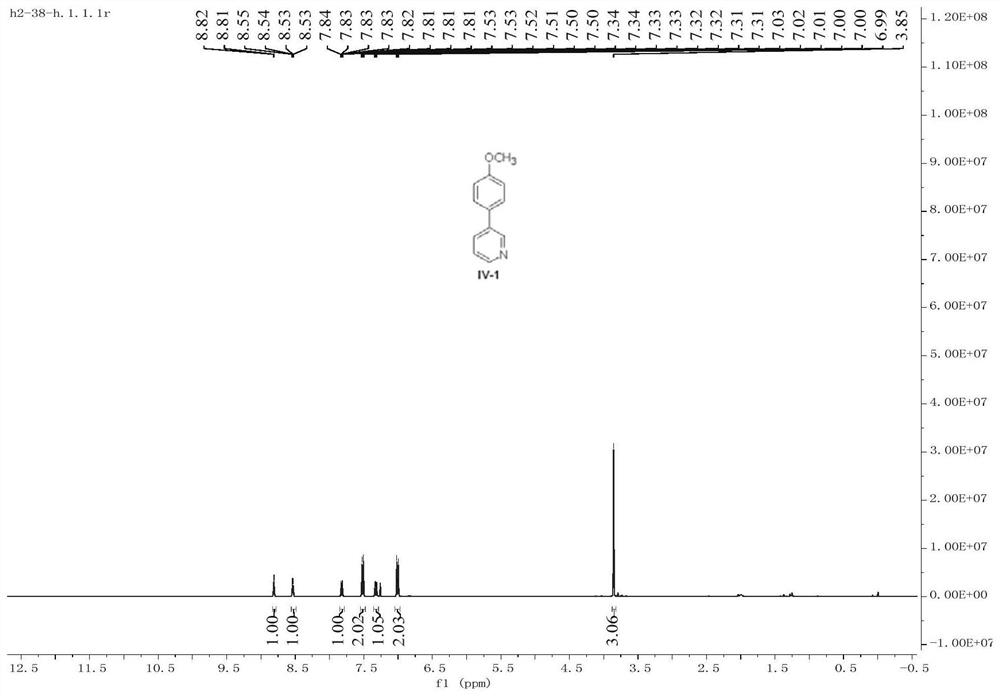
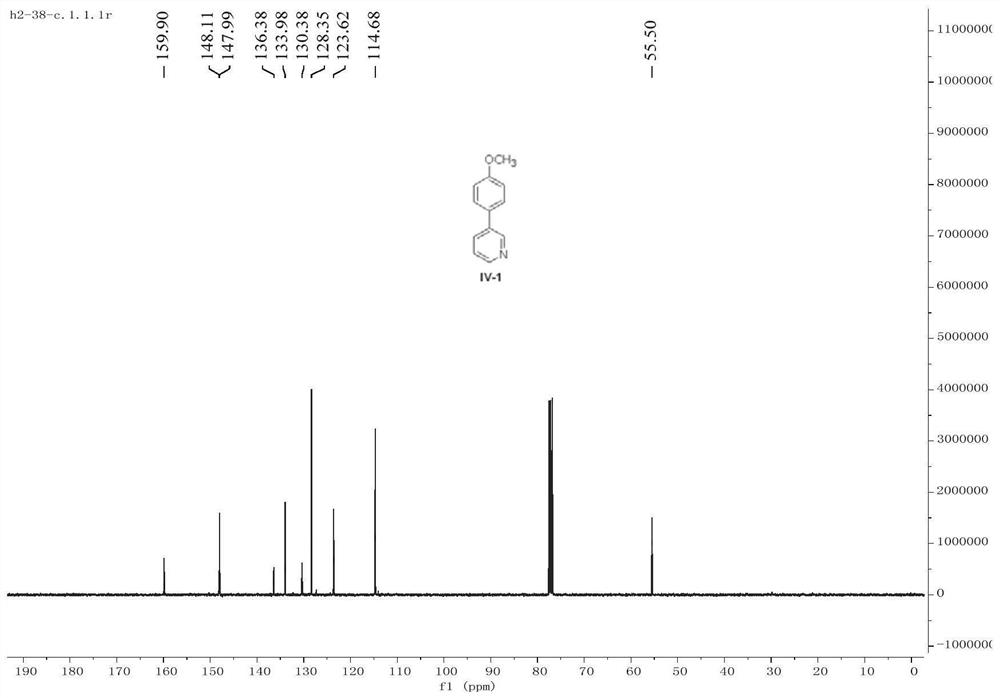
[0062] Under anhydrous and oxygen-free conditions, 3-chloropyridine II-1 (0.8 mmol) represented by formula 1 and the bimetallic organic framework [CoFe(tda)H] were added to a Schlenk flask. 2 O] (5 mol%) was dissolved in anhydrous ether (4 mL) solvent. After stirring at 0 °C for 10 min, p-methoxyphenylmagnesium bromide III-1 (1.2 mmol) was dropwise added to the reaction system, and then placed at 25 °C for 15 h. After the reaction, 3 mL of saturated ammonium chloride solution was added to the reaction system for quenching, and then 10 mL of ethyl acetate was added for extraction. The obtained organic phase was filtered and concentrated under reduced pressure to remove the solvent, and the residue was separated by column chromatography with the eluting solvent: ethyl acetate / petroleum ether to obtain the product IV-1 as a yellow oil (70% yield).
[0063] 1 H NMR (400MHz, CDCl 3 )δ8.81(d,J=2.4,1H),8.54(dd,J=4.8,1.6Hz,1H),7.84–7.81(m,1H),7.53–7.50(m,2H),7.34–7.31( ...
Embodiment 2
[0065] The solvent was tetrahydrofuran instead of ether, and other conditions were the same as those in Example 1, and the yield of the target product IV-1 was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com