Medicine for treating acute myelogenous leukemia and application thereof
A technology for acute myeloid and leukemia, applied in the field of biomedicine, can solve the problem that the role of HMA needs to be studied, and achieve the effect of enriching treatment strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
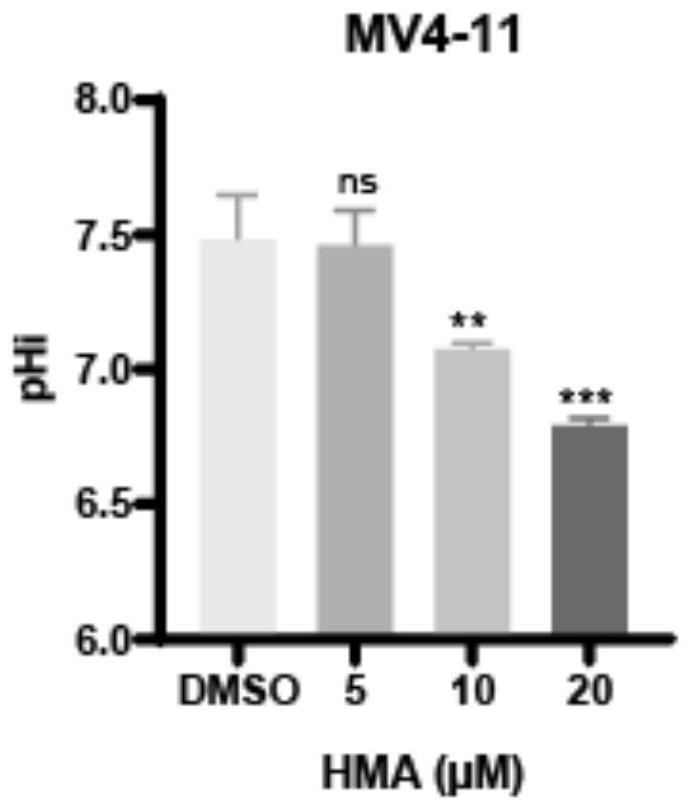
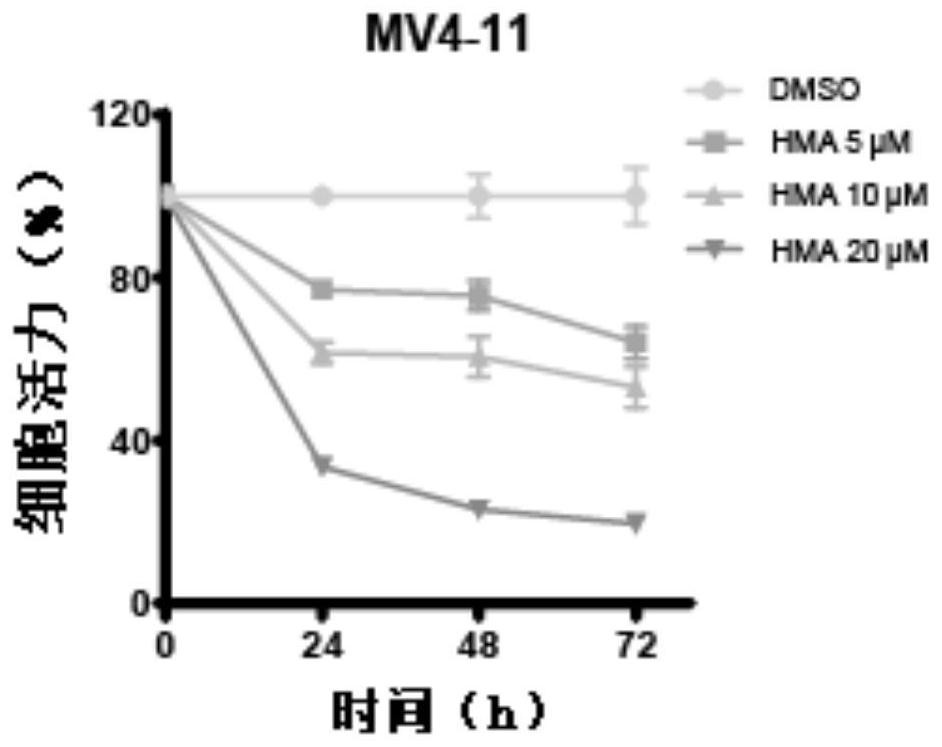
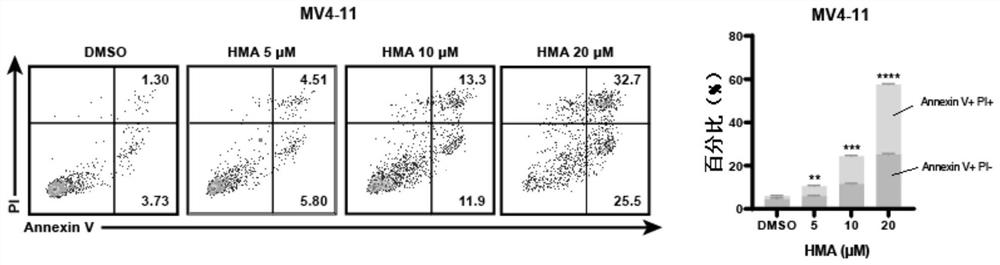
[0052] Proliferation inhibition and apoptosis promotion of NHE1 inhibitor HMA on leukemia cells:
[0053] Will 4x10 6 MV4-11 cells were seeded in a six-well plate, and different concentrations of NHE1 inhibitors were added to make the final concentrations 0, 5 μM, 10 μM, and 20 μM, respectively. Blow evenly, and put them in an incubator for 48 hours. After culturing for 48 hours, the following experiments were performed:
[0054] (1) Intracellular pH detection:
[0055] To develop a standard curve: wash cells twice with PBS, add BCECF-AM probe at a final concentration of 5 μM, and stain for 30 minutes at room temperature in the dark. Wash once with PBS, resuspend with different pH calibration buffers, add 10 μmol Nigericin and Valinomycin, and incubate for 10 minutes to make the intracellular pH reach 4.5, 5.5, 6.5, and 7.5, respectively; use a microplate reader to detect when the excitation light is 490 nm and 440nm, the emission light is the fluorescence intensity of 535nm...
Embodiment 2
[0065] Proliferation-inhibiting and apoptosis-promoting effects of HMA on primary cells of AML patients:
[0066] (1) Isolation and culture of bone marrow mononuclear cells:
[0067] Collect 5mL of fresh bone marrow samples from AML patients into a purple-headed heparin anticoagulant tube, mix the fresh bone marrow samples with an equal volume of PBS (pre-cooled at 4°C) evenly; take a clean 15mL centrifuge tube, add 3mL of human lymphocytes to the tube to separate Aspirate the above diluted bone marrow sample, tilt the centrifuge tube at 45 degrees to avoid shaking, and slowly add the bone marrow sample to the lymphocyte separation solution along the wall of the centrifuge tube, so that there is a clear separation between the separation solution and the bone marrow fluid level. Floor. The centrifuge tube was placed in a centrifuge and centrifuged at 2000 rpm for 20 minutes. Gently take out the centrifuge tube, avoid shaking, take another 15mL sterile centrifuge tube and add ...
Embodiment 3
[0074] The combined application of HMA and venetoclax inhibits the proliferation and promotes apoptosis of leukemia cells:
[0075] Will 4x10 6 MV4-11 cells were seeded in a six-well plate, and were given no drug, 0.1 μM venetoclax, 10 μM HMA and the combination of the two drugs; they were blown evenly and placed in an incubator for 48 hours to conduct the following experiments:
[0076] (1) Cell viability detection:
[0077] MV4-11 cells were plated at 5 x 10 4 Cells / mL were seeded in 96-well plates. Cells with different treatments were set to 3-4 sub-wells, and the volume of each well was 100 μL. Put it into a cell incubator and cultivate for 48 hours; add 10 μl of CCK8 working solution to each well, mix well, and continue to incubate in the dark in the incubator for 3 hours, and then use a microplate reader to detect the absorbance at a wavelength of 450 nm. The result is as Figure 7 shown, Figure 7 It shows that both venetoclax and HMA can significantly inhibit the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com