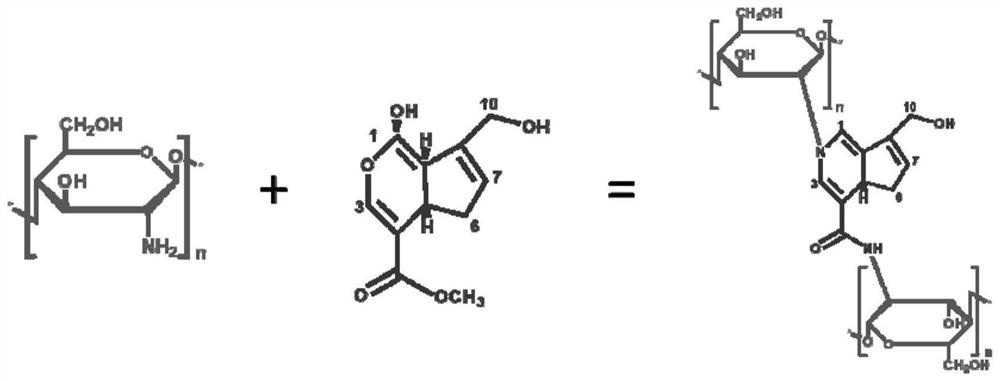
Monodisperse gelatin chitosan composite embolism microsphere with controllable degradation performance and elasticity and preparation method of monodisperse gelatin chitosan composite embolism microsphere
A monodisperse, gelatin shell technology, applied in the field of biomedicine, can solve the problems of poor sphericity, uncontrollable degradability and elasticity, poor biocompatibility, etc. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In the present embodiment, the preparation of monodisperse gelatin-chitosan composite embolization microspheres is as follows:
[0056] (1) Preparation of inner phase, outer phase fluid and collection solution
[0057] Preparation of internal phase fluid: add gelatin and chitosan into acetic acid aqueous solution with a concentration of 0.32 mol / L, stir in a water bath at 40 ° C until completely dissolved, then cool to room temperature, put it in a vacuum drying box and vacuumize at room temperature to Remove the bubbles in the solution to obtain an internal phase fluid; in the internal phase fluid, the mass ratio of acetic acid aqueous solution, gelatin, and chitosan is 1:0.1:0.02;
[0058] Preparation of external phase fluid: dissolving oil-soluble surfactant Span80 in n-octanol to obtain an external phase fluid; in the external phase fluid, the mass ratio of n-octanol to Span80 is 1:0.04.
[0059] Preparation of collection solution: dissolving cross-linking agent Ge...
Embodiment 2
[0071] In the present embodiment, the preparation of monodisperse gelatin-chitosan composite embolization microspheres is as follows:
[0072] (1) Preparation of inner phase, outer phase fluid and collection solution
[0073] The same as the inner phase fluid, outer phase fluid and collection liquid in Example 1.
[0074] (2) Preparation of monodisperse gelatin-chitosan composite embolization microspheres
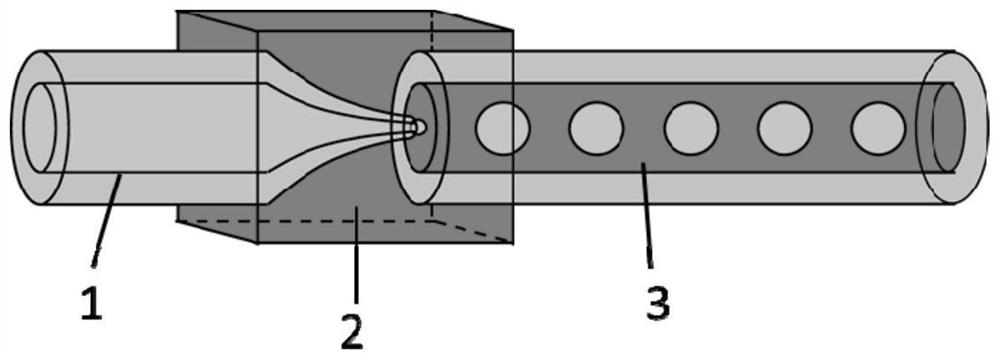
[0075] using a structure such as figure 2 The microfluidic device shown, the inner diameter of the outlet of the injection tube is 60 μm, and the inner diameter of the collection tube is 300 μm. The temperature of the constant humidity box was 40°C.
[0076] The inner phase fluid is input into the injection tube of the microfluidic device with a syringe pump, and the outer phase fluid is input into the collection tube of the microfluidic device with a syringe pump, and a monodispersed W / O emulsion is formed in the collection tube, using a syringe filled with collection ...
Embodiment 3
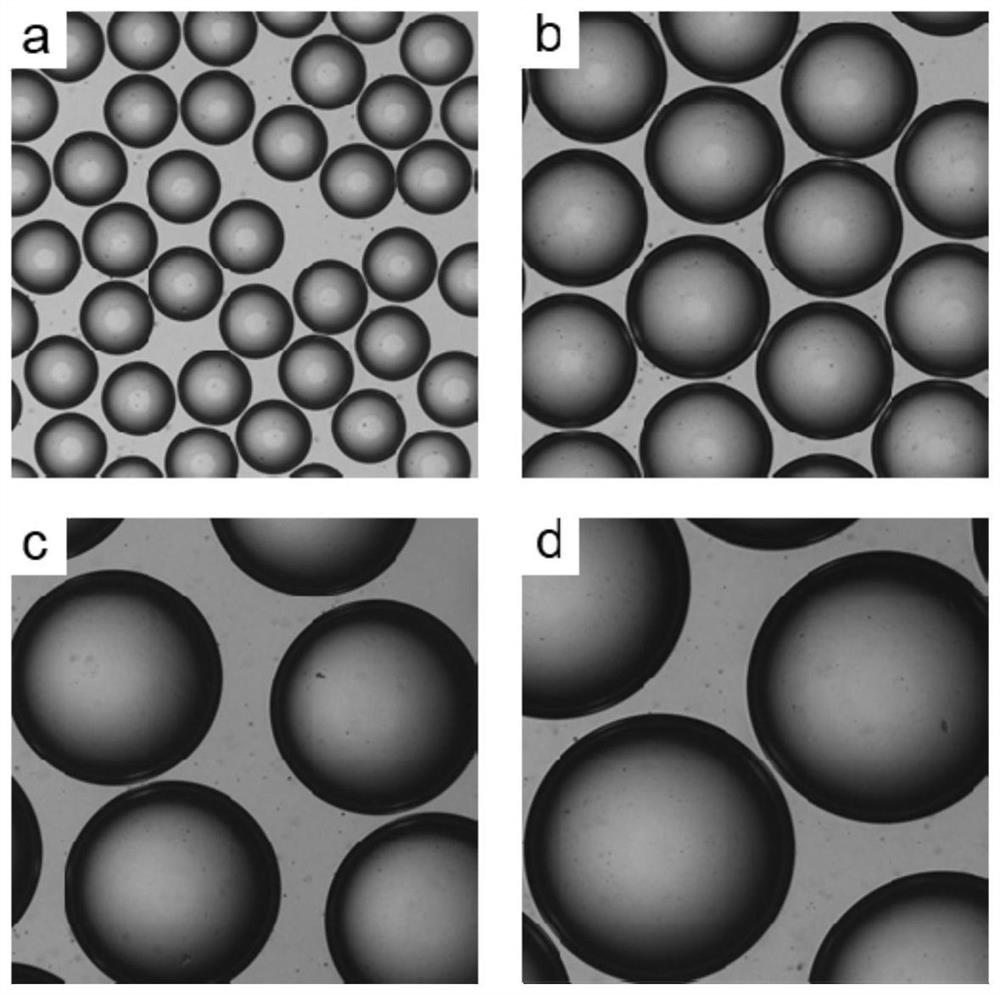
[0083] In the present embodiment, investigate the influence of the content of genipin in the collection solution on the preparation of gelatin-chitosan plug composite plug microspheres, and the steps are as follows:
[0084] (1) Preparation of inner phase, outer phase fluid and collection solution
[0085] The inner phase fluid and outer phase fluid are the same as in Example 1. The following four collection solutions with different genipin contents were prepared:
[0086] Different amounts of genipin were dissolved in the same amount of n-octanol to obtain collection solutions A, B, C, D; in collection solution A, the mass ratio of n-octanol and genipin dissolved was 1:0.0025; In solution B, the mass ratio of n-octanol and genipin solution is 1:0.005; in collection solution C, the mass ratio of n-octanol and genipin solution is 1:0.0075; in collection solution D, n-octanol and The mass ratio of genipin is 1:0.01.
[0087] (2) Preparation of gelatin-chitosan composite embol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com