Verification method for calculating atmospheric oxidative degradation path of mesitylene by adopting high-level quantum chemistry
A technology of quantum chemical calculation and mesitylene, applied in the field of quantum chemical calculation, can solve problems such as poor mesitylene reproducibility, and achieve the effect of solving poor reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
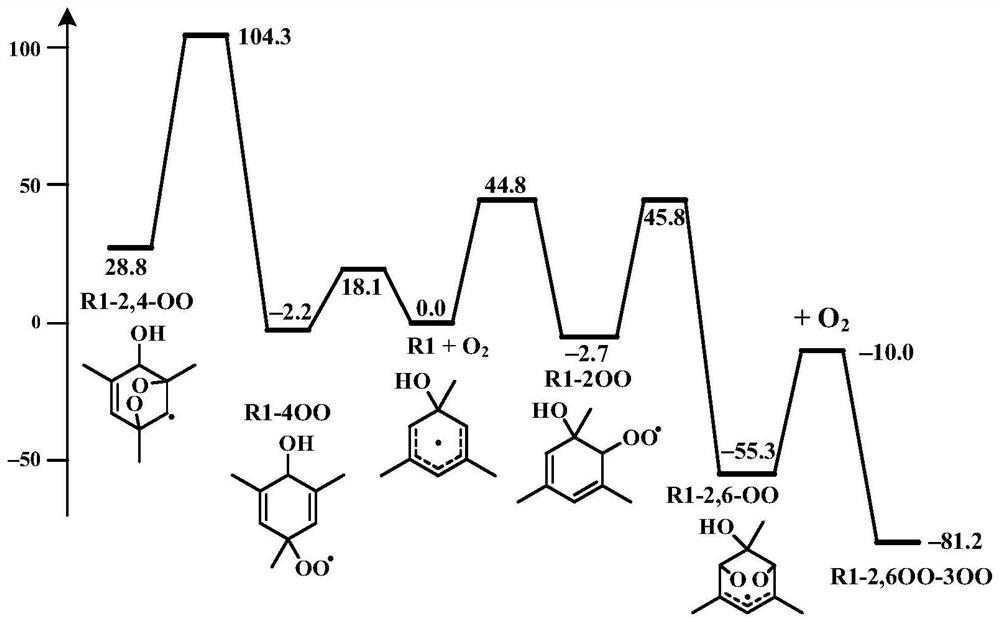
[0028] Here, the R1 radical generated in situ by the addition of OH radical to the methyl group is taken as an example. The present invention uses Gaussian 09 program to calculate the transition state of the R1 radical reaction path, the structure optimization method is M062X / 6-311++G(2df, 2p), and the single-point energy calculation method is CBS-QB3. At the same time, the present invention uses the transition state theory to pass the Gibbs free energy change Δ of the reaction r G ≠ The reaction rate constant k(T) can be calculated by the following formula:
[0029]
[0030] where κ is the tunneling correction factor, k b is the Boltzmann constant, Δ r G ≠ is the Gibbs free energy difference between the transition state and the reactant, n=1, 2 represents the unimolecular and bimolecular reactions, respectively. Whether or not the reaction can occur can be confirmed by the value of the reaction rate constant. figure 1 The calculated results of the transition state of...
Embodiment 2
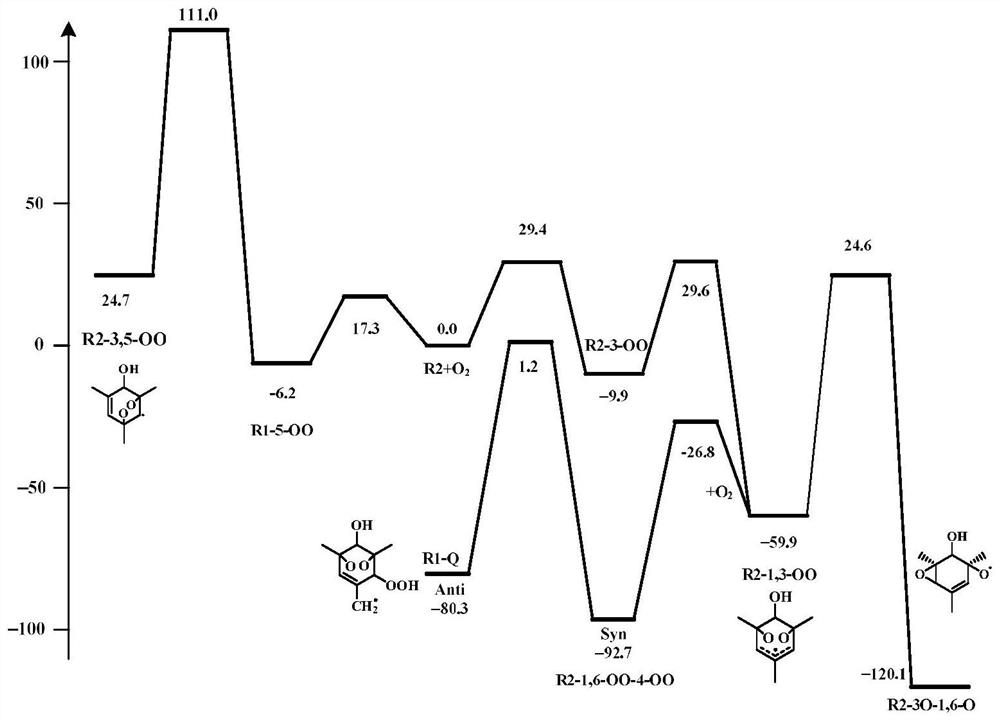
[0038]Here, the R2 radical generated by the addition of OH radical at the ortho position of the methyl group is taken as an example. The present invention uses Gaussian 09 program to calculate the transition state of the R2 radical reaction path, the structure optimization method is M062X / 6-311++G(2df, 2p), and the single-point energy calculation method is CBS-QB3. At the same time, the present invention uses the transition state theory to combine the Gibbs free energy change Δ of the reaction r G ≠ To calculate the reaction rate constant k(T), the formula for calculating the adiabatic ionization energy can be expressed as:
[0039]
[0040] where κ is the tunneling correction factor, k b is the Boltzmann constant, Δ r G ≠ is the Gibbs free energy difference between the transition state and the reactant, n=1, 2 represents the unimolecular and bimolecular reactions, respectively. Whether or not the reaction can occur can be confirmed by the value of the reaction rate co...
Embodiment 3
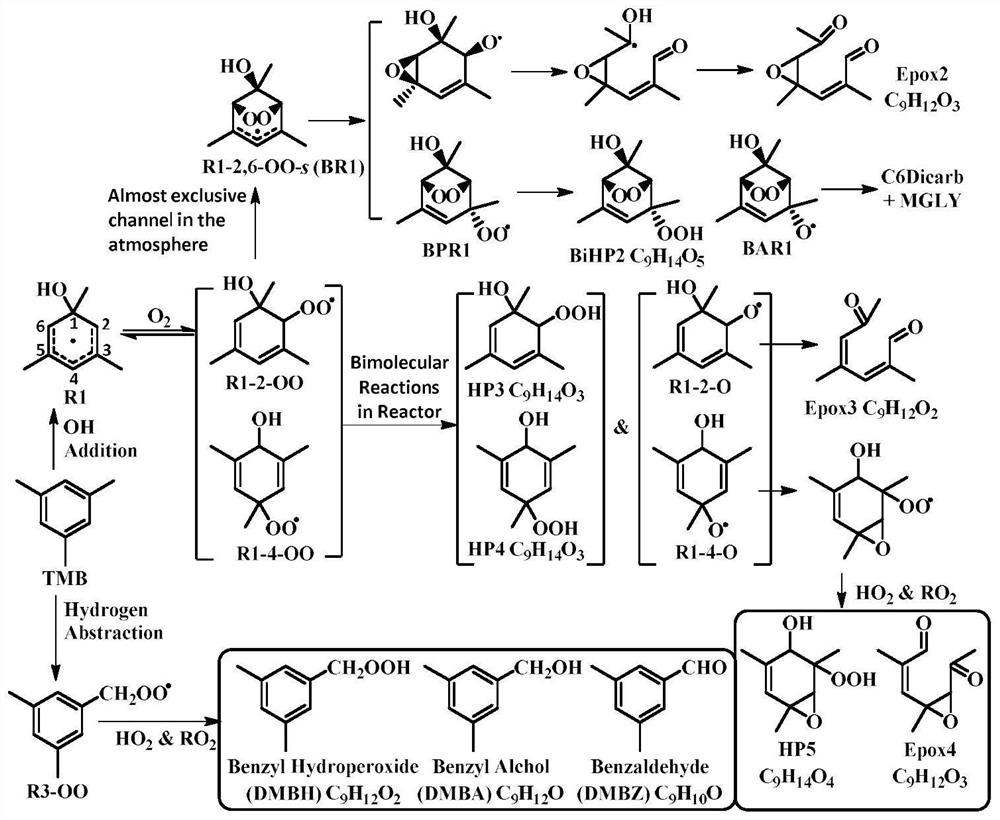
[0047] Here, the R3 radical generated by the OH radical abstracting the hydrogen on the mesityl group is taken as an example. The R3 radical reaction process is relatively simple, and there are many bimolecular reactions, so there is no need to perform transition state calculations here. Figure 4 The oxidation mechanism of the R3 radical is shown. After the R3 radical is formed, it will quickly combine with oxygen to generate the R3-OO radical, and then the R3-OO radical will react with HO. 2 , RO 2 and NO X Such free radicals undergo bimolecular reactions and generate DMBA, DMBZ and DMBH. At the same time, in order to verify the correctness of the mechanism, the invention is verified by adiabatic ionization energy calculation combined with simulation experiments. The adiabatic ionization energy calculation formula can be expressed as:
[0048] ΔE=E cation -E netural
[0049] where ΔE is the value of adiabatic ionization energy, E cation is the energy of the cation aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com