Cobalt-based oxygen reduction electro-catalytic material and preparation method thereof
An electrocatalytic material, cobalt-based technology, used in circuits, electrical components, battery electrodes, etc., can solve the problems of cumbersome preparation, low catalytic activity, and low utilization of active sites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) The ZIF-67 was placed in a quartz boat, and placed in a vacuum tube furnace with nitrogen, heated to 800°C at a heating rate of 10°C / min, kept for 2 hours, and then cooled to room temperature with the furnace to obtain the intermediate product;
[0021] (2) After fully mixing the intermediate product with the sulfur powder (the mass ratio is 1:3), place it in a quartz boat, and place it in a tube furnace with nitrogen, and heat it up to 155°C at a heating rate of 10°C / min , kept for 30 min; then heated to 300 °C at a heating rate of 10 °C / min, held for 2 h, and cooled to room temperature with the furnace to obtain a cobalt-based oxygen reduction electrocatalytic material.
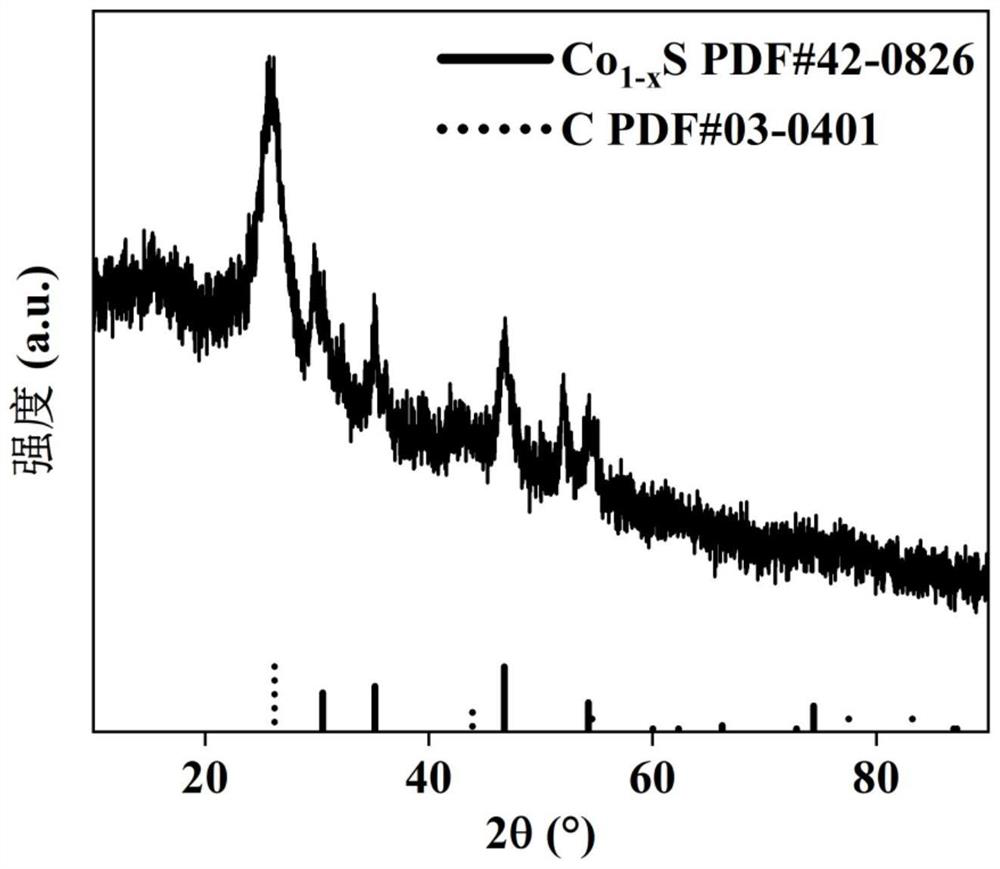
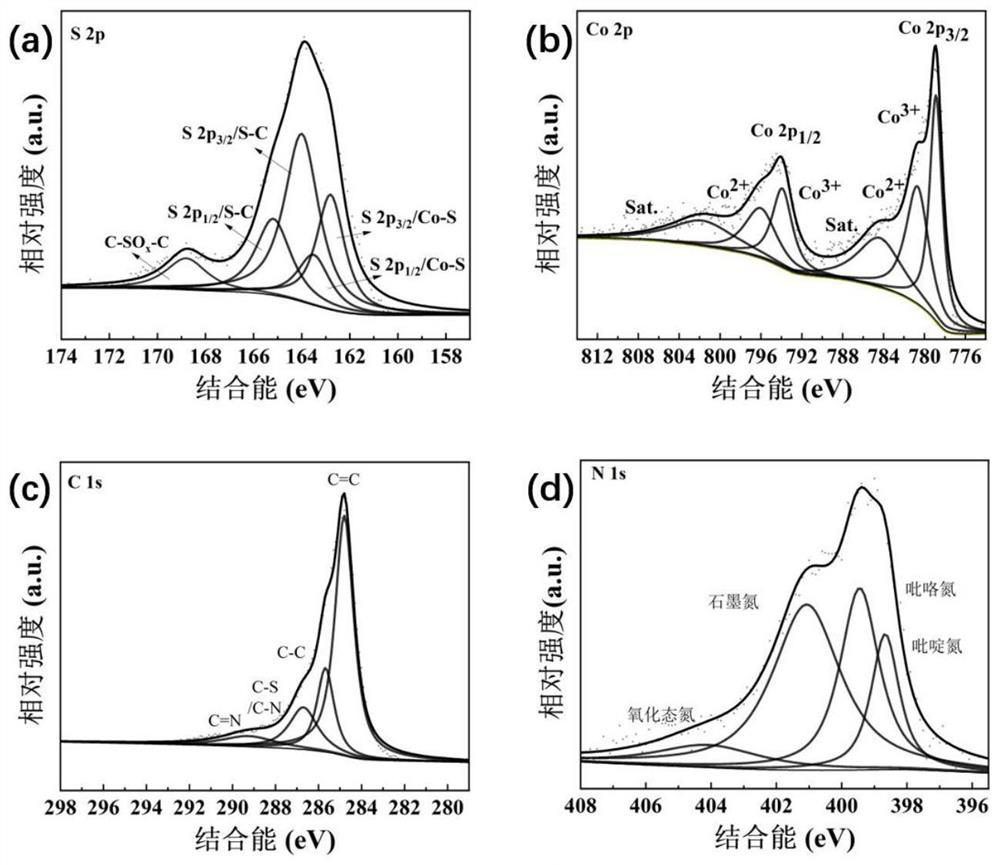
[0022] XRD analysis of the cobalt-based electrocatalytic material for oxygen reduction reaction synthesized in Example 1 shows that the phase composition of the material is Co 1-x S@C, such as figure 1 shown; and then using ICP analysis to obtain that the cobalt-sulfur atomic molar ratio of the...
Embodiment 2
[0031] (1) The ZIF-67 was placed in a quartz boat, placed in a vacuum tube furnace with nitrogen, heated to 750°C at a heating rate of 8°C / min, kept for 1.5h, and then cooled to room temperature with the furnace to obtain mid product;
[0032] (2) After fully mixing the intermediate product with the sulfur powder (mass ratio is 1:2.5), place it in a quartz boat, and place it in a tube furnace with nitrogen, and heat it up to 145°C at a heating rate of 8°C / min , kept for 20 min; then heated to 290 °C at a heating rate of 8 °C / min, kept for 1 h, and cooled to room temperature with the furnace to obtain a cobalt-based oxygen reduction electrocatalytic material.
[0033] Using ICP analysis, the cobalt-sulfur atomic molar ratio of the material prepared in Example 2 was 0.46, that is, Co 1-x x=0.54 in S. Figure 5 For the pore and specific surface area diagram of the synthetic material in Example 2, the specific surface area of the material is 97.19m 2 g -1 , with an average ...
Embodiment 3
[0035] (1) The ZIF-67 was placed in a quartz boat, and placed in a vacuum tube furnace with nitrogen, heated to 820°C at a heating rate of 10°C / min, kept for 3 hours, and then cooled to room temperature with the furnace to obtain the intermediate product;
[0036] (2) After fully mixing the intermediate product with the sulfur powder (the mass ratio is 1:4), place it in a quartz boat, and place it in a tube furnace with nitrogen, and heat it up to 165°C at a heating rate of 10°C / min , kept for 45 min; then heated to 320 °C at a heating rate of 8 °C / min, kept for 3 h, and cooled to room temperature with the furnace to obtain a cobalt-based oxygen reduction electrocatalytic material.
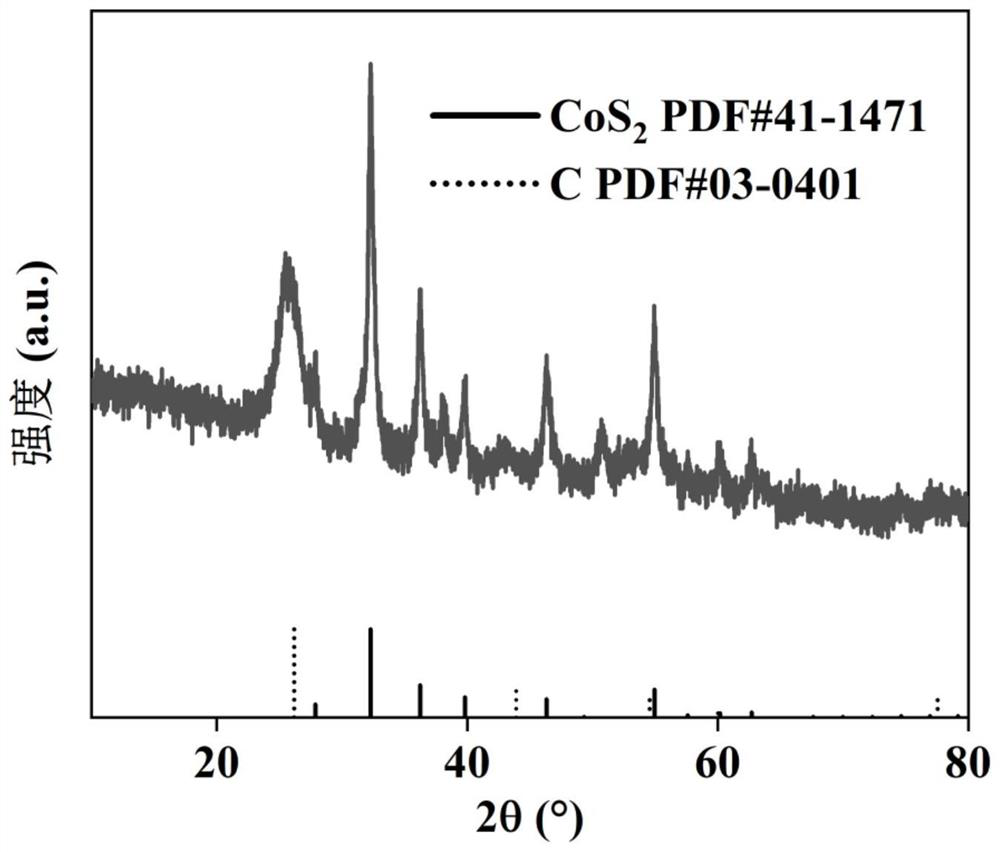
[0037] Using ICP analysis, the cobalt-sulfur atomic molar ratio of the material prepared in Example 3 was 0.43, that is, Co 1-x x=0.57 in S.
[0038] In order to evaluate the oxygen reduction catalytic ability of the material, a lithium-oxygen battery was assembled with the cobalt-based oxygen r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com