Cell imaging composition and cell substance imaging method using same
A technology of cell imaging and imaging methods, applied in biochemical equipment and methods, chemical instruments and methods, microbial measurement/inspection, etc., can solve the problems of complex labeling operations, changing the structure of chemical labels, etc., and achieve excellent selectivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148]
[0149]
[0150] 2-Aminobenzophenone (2-Aminobenzophenone) (2.00g, 10.1mmol), 2-bromomethylphenyl boronic acid (2.59g, 12.1mmol), potassium carbonate (potassiumcarbonate) ( 1.39 g, 10.1 mmol) was added to acetonitrile solvent (15 mL) and refluxed for 8 hours. After drying the reaction solution using an evaporator, Compound 1 (2.5 g, 7.6 mmol) was obtained through a silica gel column using EA / hexane (1 / 4) as a developer immediately.
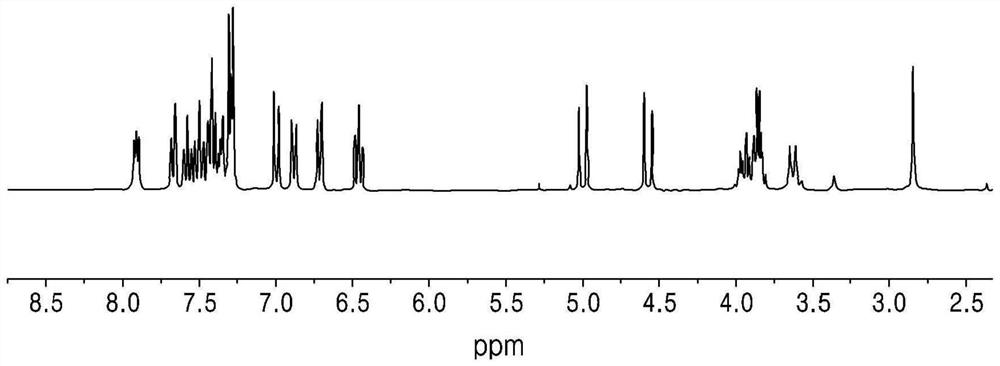
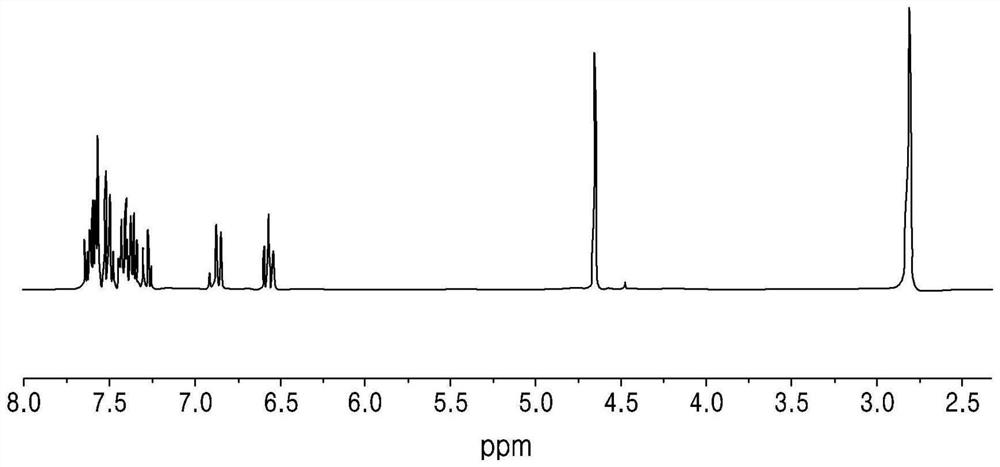
[0151] Yield: 76%. 1 H NMR (300MHz, CD 3 CN): δ7.64-7.61(m, 1H), 7.60-7.59(m, 1H), 7.58-7.55(m, 2H), 7.53-7.52(m, 1H), 7.51-7.47(m, 1H), 7.43-7.39(m, 2H), 7.38-7.33(m, 2H), 7.30-7.25(m, 1H), 6.88(d, 1H, 9Hz), 6.59-6.54(m, 1H), 4.65(s, 2H) ). 13 C NMR (125MHz, CDCl 3 ): 199.58, 151.38, 143.75, 140.62, 135.42, 135.20, 134.56, 131.24, 129.91, 129.11, 128.39, 128.01, 126.77, 114.63, 112.68, 46.99. HRMS(EI): for C 20 H 19 N 1 O 1 B 1 [M+H] + Calculated: 332.1850; Found: 332.2168.
Embodiment 2
[0152]
[0153] Instrument used: Scinco FS-2
[0154] Concentration of compound 1: 1.5mM
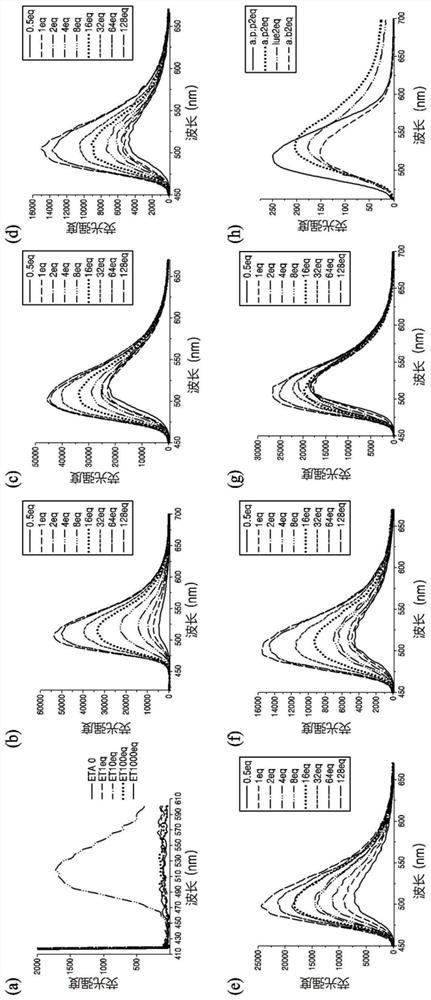
[0155] result: figure 1 .
[0156] refer to figure 1 The fluorescence spectrum of compound 1 as a probe can be confirmed: (a) compound 1 + ethanolamine, (b) compound 1 + alaninol, (c) compound 1 + phenylalaninol, (d) Compound 1 + Aminobutanol, (e) Compound 1 + Valinol, (f) Compound 1 + Leucinol, (g) Compound 1 + Tryptophanol, and (h) Comparative (Compound 1 + Alaninol, Leucinol) Amino alcohol, amino butanol, phenylalaninol) (can be omitted).
Embodiment 3
[0157]
[0158] The instruments, concentrations and parameters used were the same as in the case of ethanolamine and alaninol.
[0159] result: figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com