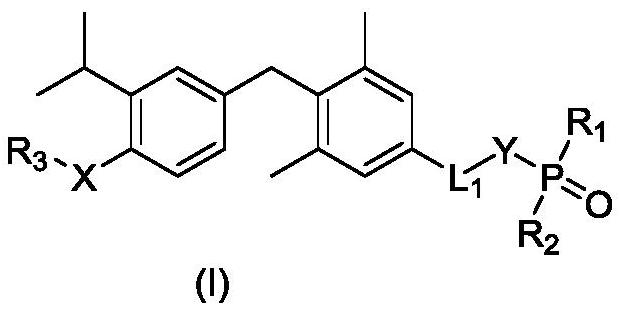
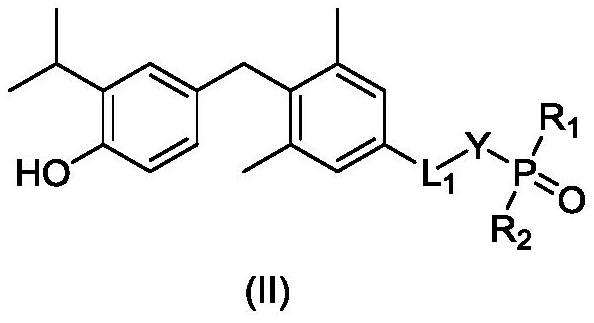
Phosphoric acid or phosphate ester derivative as well as preparation method and medical application thereof
A technology for medicines and compounds, applied in the fields of phosphoric acid or phosphoric acid ester derivatives and their preparation and their use in medicine, can solve problems such as effectiveness, safety or selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
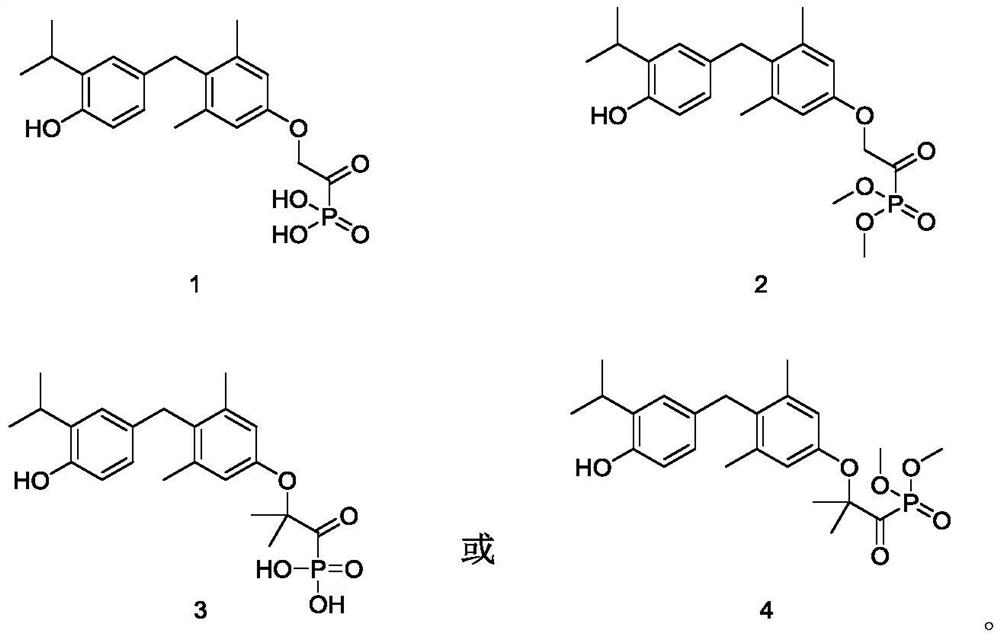
Embodiment 1 and Embodiment 2
[0063]
[0064] first step
[0065] (4-(4-(Benzyloxy)-3-isopropylbenzyl)-3,5-dimethylphenoxy)triisopropylsilane
[0066] At room temperature, (4-(benzyloxy)-3-isopropylphenyl)(2,6-dimethyl-4-((triisopropylsilyl)oxy)phenyl)methanol 1a ( Prepared by the well-known method "Journal of the American Chemical Society, 2006, vol.128, #27, p.8868-8874") 15.0 g (28.2 mmol) was dissolved in 100 mL of dichloromethane, and triethylsilane was added 8.9 mL (56.3 mmol), 320 mg (2.8 mmol) of trifluoroacetic acid, and reacted at room temperature for 12 hours. The reaction solution was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography with eluent system B to obtain the title product 1b (13.8 g, yellow liquid), yield: 95.1%
[0067] 1 H NMR(400MHz, CHLOROFORM-d)δppm 1.09-1.15(m,18H)1.18(d,J=6.85Hz,6H)1.23-1.34(m,3H)2.19(s,6H)3.37(spt,J=6.89 Hz,1H)3.92(s,2H)5.03(s,2H)6.61(s,2H)6.65-6.72(m,1H)6.77(d,J=8.31Hz,1H)6.93(d,J=2.20Hz, 1...
Embodiment 3 and Embodiment 4
[0089]
[0090] first step
[0091] (4-(4-(Benzyloxy)-3-isopropylbenzyl)-3,5-dimethylphenoxy)triisopropylsilane
[0092] Using the synthetic route of the first step of Example 1 and Example 2, 3b was obtained.
[0093] second step
[0094] 4-(4-(Benzyloxy)-3-isopropylbenzyl)-3,5-dimethylphenol
[0095] Using the synthetic route of the second step of Example 1 and Example 2, 3c was obtained.
[0096] third step
[0097] Ethyl 2-(4-(4-(benzyloxy)-3-isopropylbenzyl)-3,5-dimethylphenoxy)-2-methylpropanoate
[0098] The synthetic route of Example 1 and Example 2 is adopted, except that the raw material ethyl bromoacetate in the third step of Example 1 and Example 2 is replaced with ethyl 2-bromoisobutyrate to obtain the title product 3d.
[0099] the fourth step
[0100] 2-(4-(4-(Benzyloxy)-3-isopropylbenzyl)-3,5-dimethylphenoxy)-2-methylpropionic acid
[0101] The synthetic route of Example 1 and Example 2 is adopted, except that the raw material 1d in the fourth step of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com