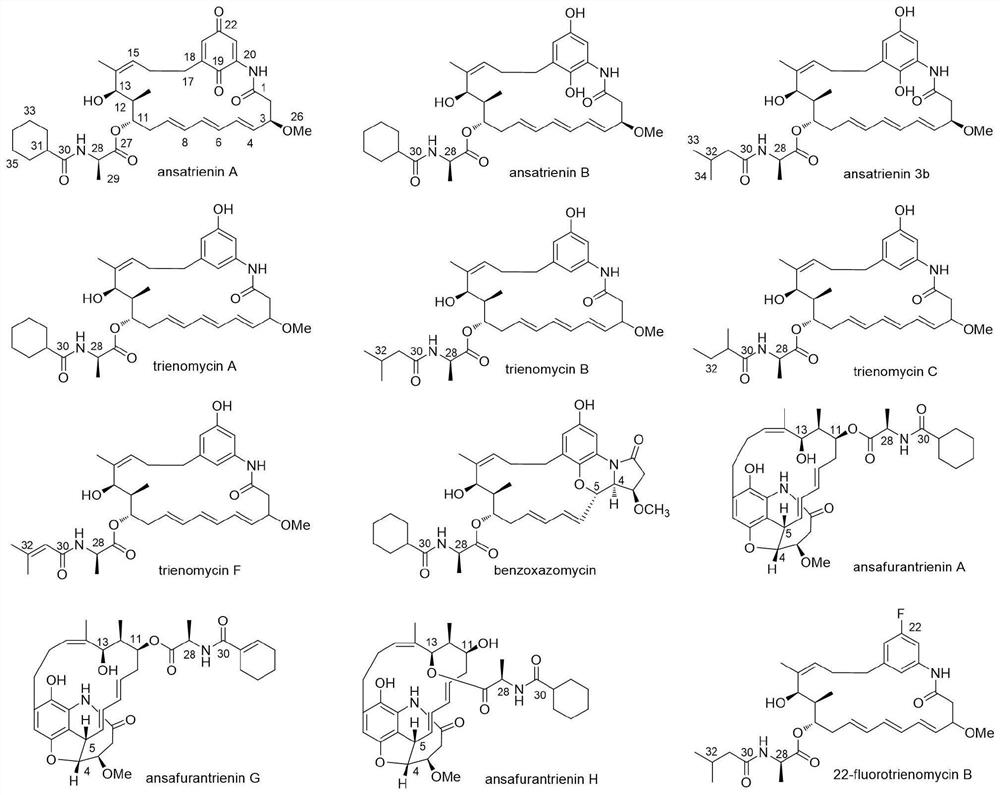
Application of ansatriene compound in antiviral infection medicine and preparation method of ansatriene compound
A technology of ansatriene and virus infection, which is applied in the field of medicine and can solve problems such as no vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] In the following examples, the experimental methods without specific conditions are usually in accordance with conventional conditions, or in accordance with the conditions suggested by the manufacturer. Unless otherwise specified, the reagents used in the examples of the present invention can be obtained from sales companies.
[0086] The Streptomyces flaveolus strain used in the present invention was purchased from the German Collection of Microorganisms, with the deposit number: DSM 9954.
[0087] The preparation method of ansatriene compound comprises the following steps:
[0088] Fermentation: Streptomyces flaveolus DSM 9954 strain was inoculated into seed medium (4% starch, 1% soy peptone, 2.1% malt extract, pH 7.0) for two days, and then the seed culture was inoculated into 30 liters of fermentation medium (4 % starch, 1% glucose, 2% soybean flour, pH 6.8) for fermentation, and cultured at 28° C. and 220 rpm for 7 days to obtain a fermented product.
[0089] Ex...
Embodiment 2
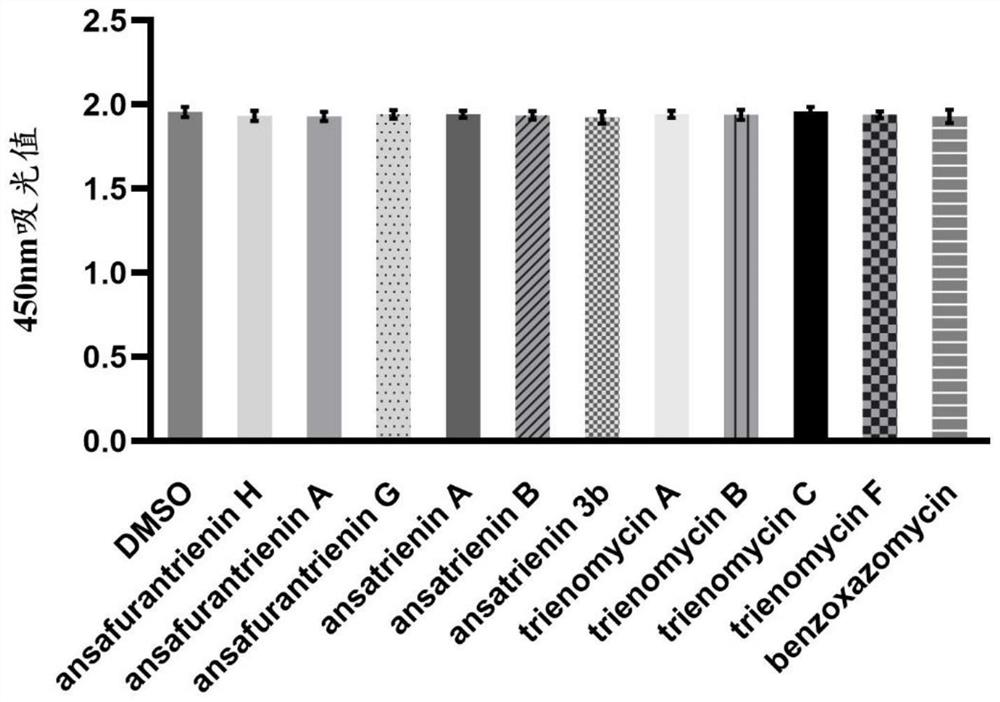
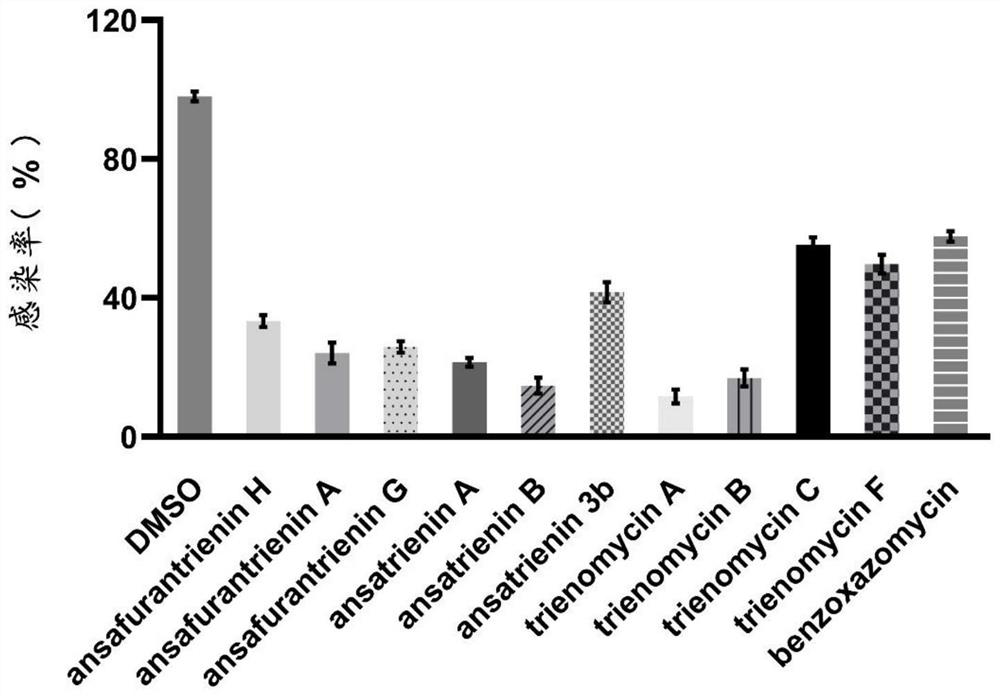
[0112] The in vivo and in vitro activity determination of the above-mentioned ansatriene compounds of the present invention against SARS-CoV-2 virus, tick-borne encephalitis virus, West Nile virus, yellow fever virus and chikungunya virus.
[0113] 1. Viruses, drugs, reagents and other materials
[0114] 1. Virus: The SARS-CoV-2 virus was isolated and cultured from the nasopharyngeal swab samples of COVID-19 patients by the Department of Biomedical Protection, Naval Medical University, and its gene sequence can be found in GenBank Accession No.MT622319. Tick-borne encephalitis virus and yellow fever virus were isolated and amplified by the Department of Biomedical Protection, Naval Medical University. West Nile virus and chikungunya virus were synthesized by reverse genetics and amplified from baby hamster kidney BHK cells. nourish. All experimental manipulations involving viral infection were performed in the P3 laboratory of the Naval Medical University.
[0115] 2. Compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com