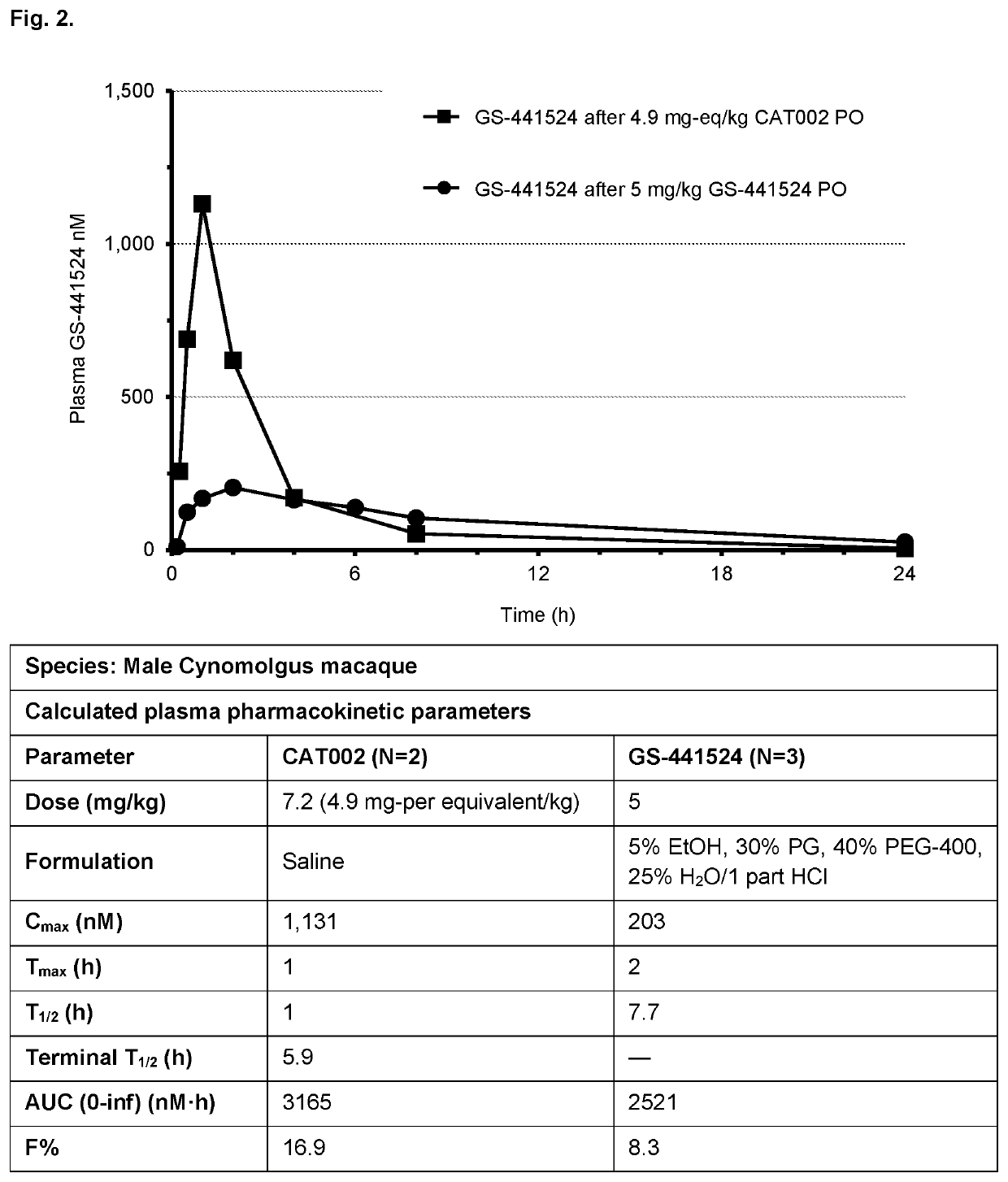
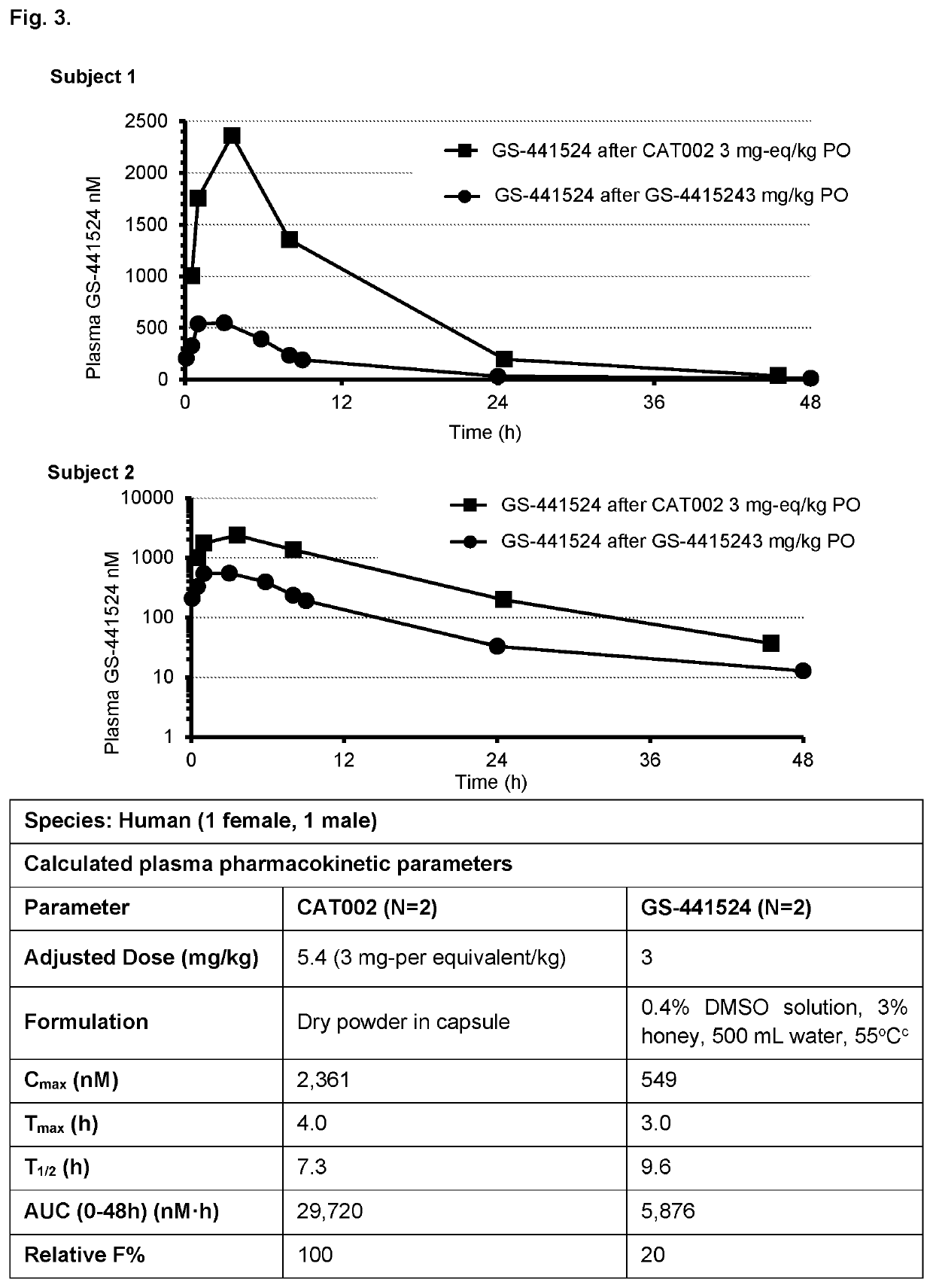
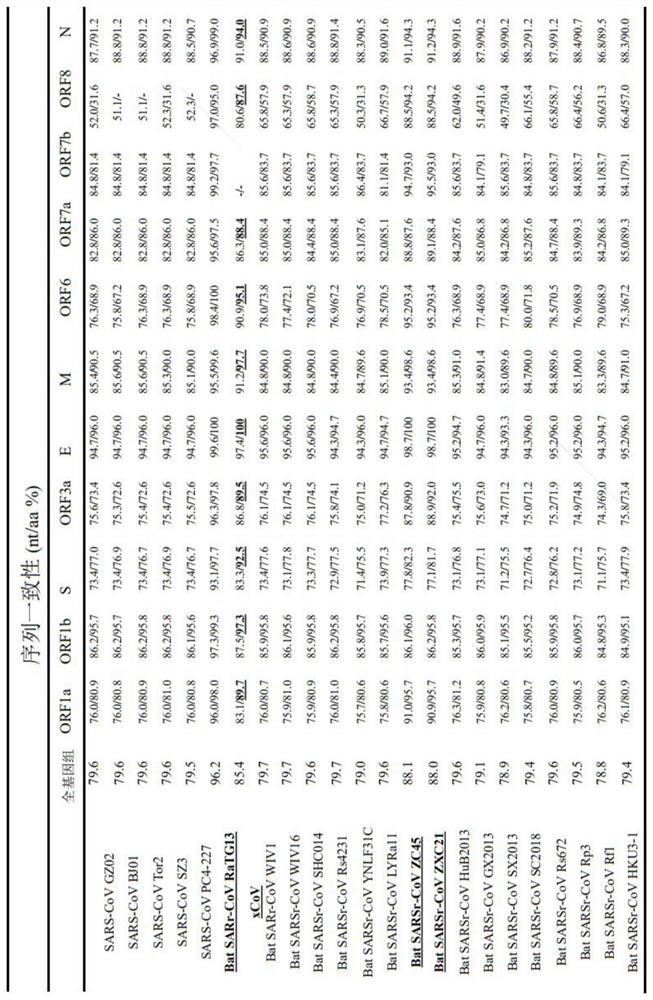
Patents
Literature
31 results about "Severe acute respiratory syndrome coronavirus RNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
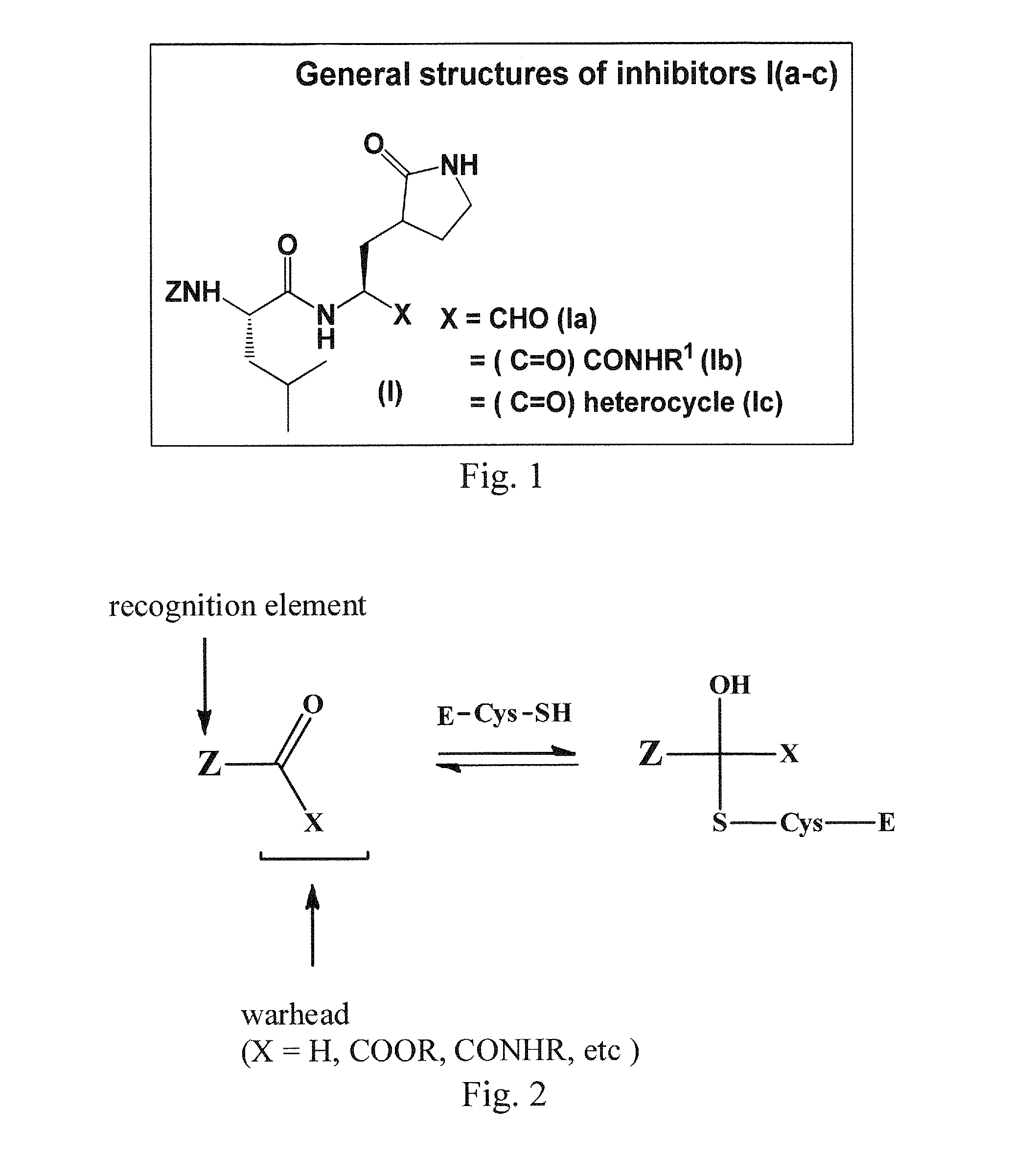
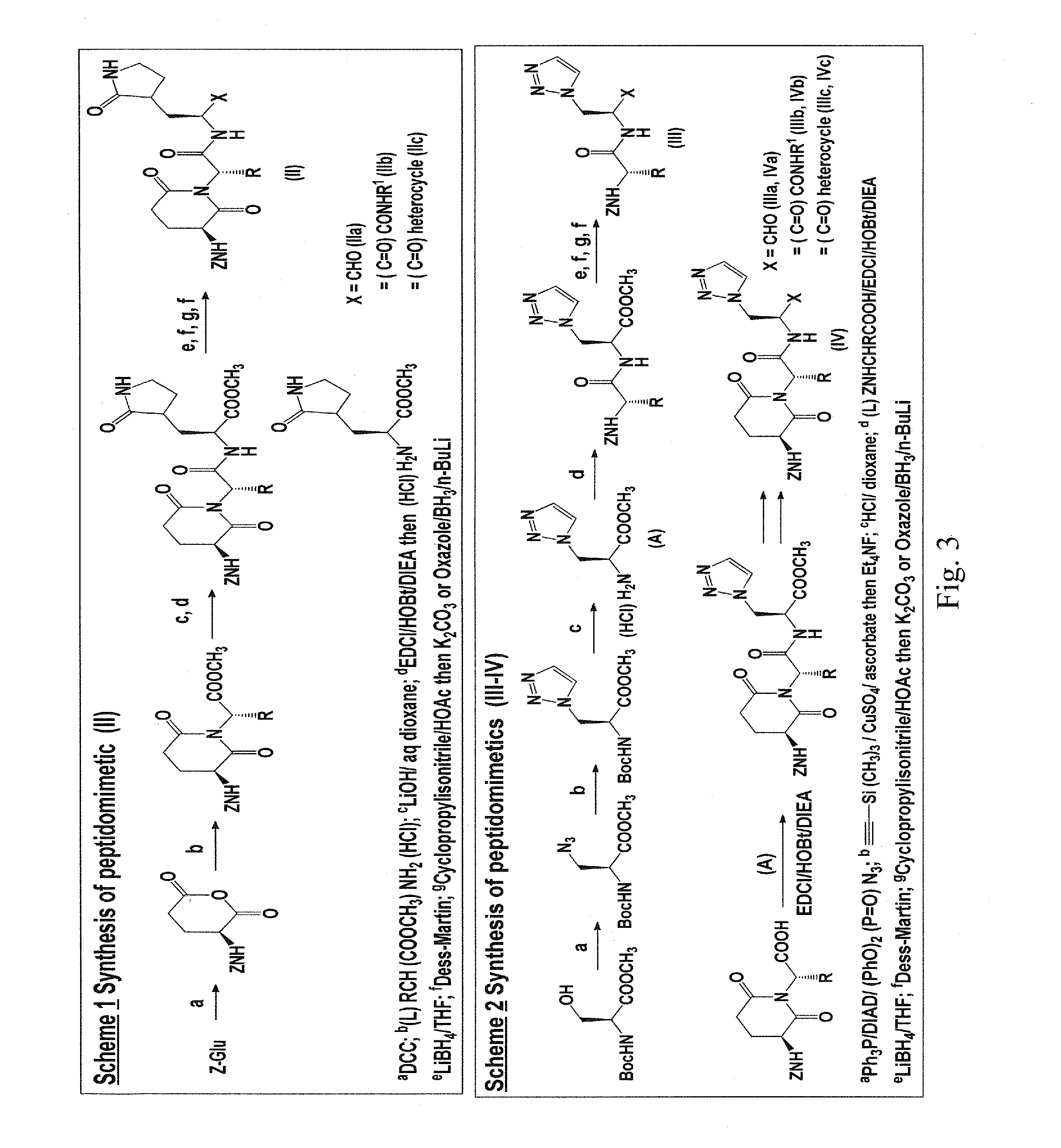
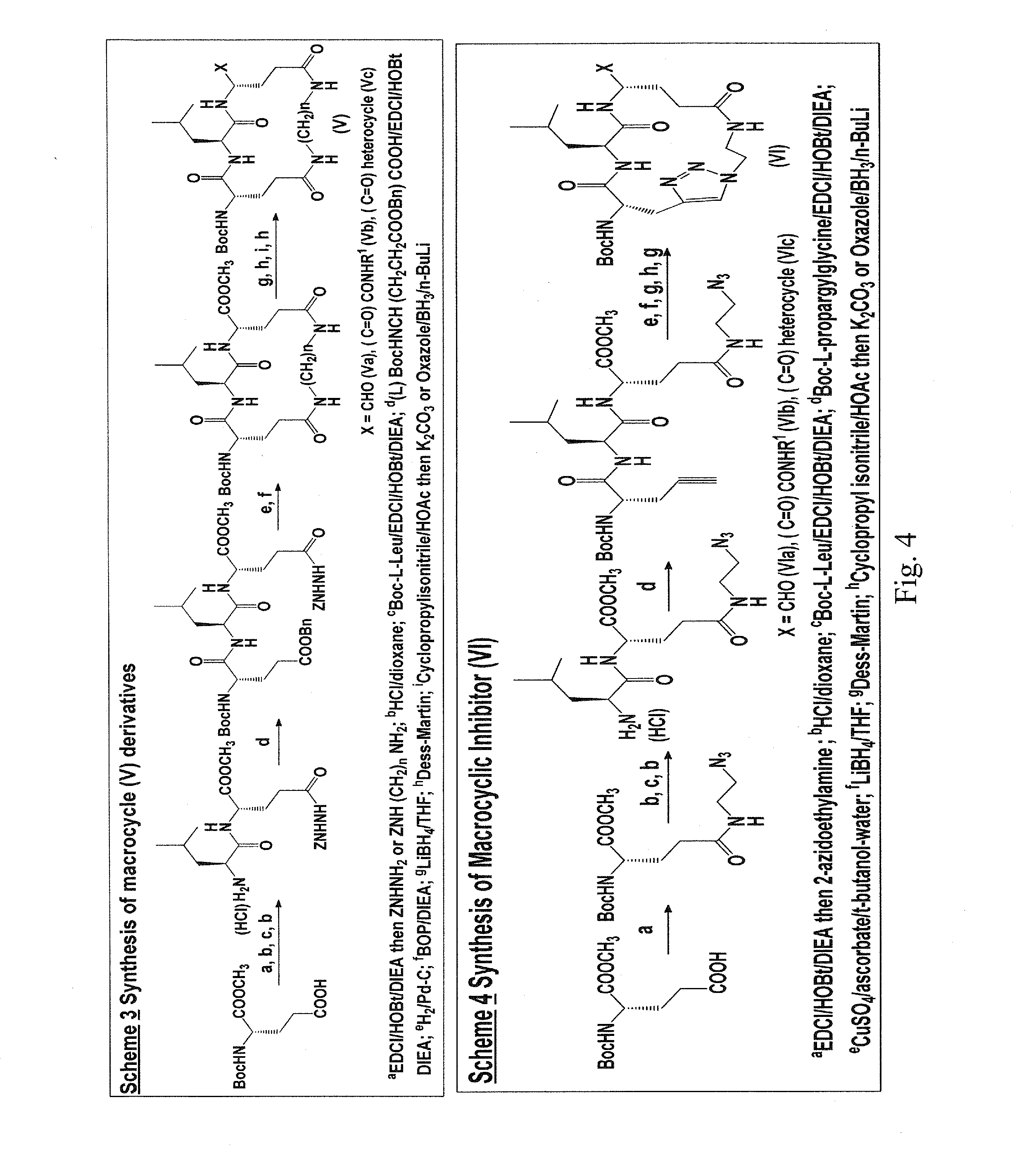
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Reagents and Methods for Detecting Severe Acute Respiratory Syndrome Coronavirus
InactiveUS20080044814A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSevere acute respiratory syndrome coronavirusPediatrics
This invention provides reagents and methods for detecting the severe acute respiratory syndrome coronavirus (SARS CoV). This invention also provides related compositions, kits, systems, and computers.
Owner:GENOME INST OF SINGAPORE
Methods and compositions for infectious cDNA of SARS coronavirus
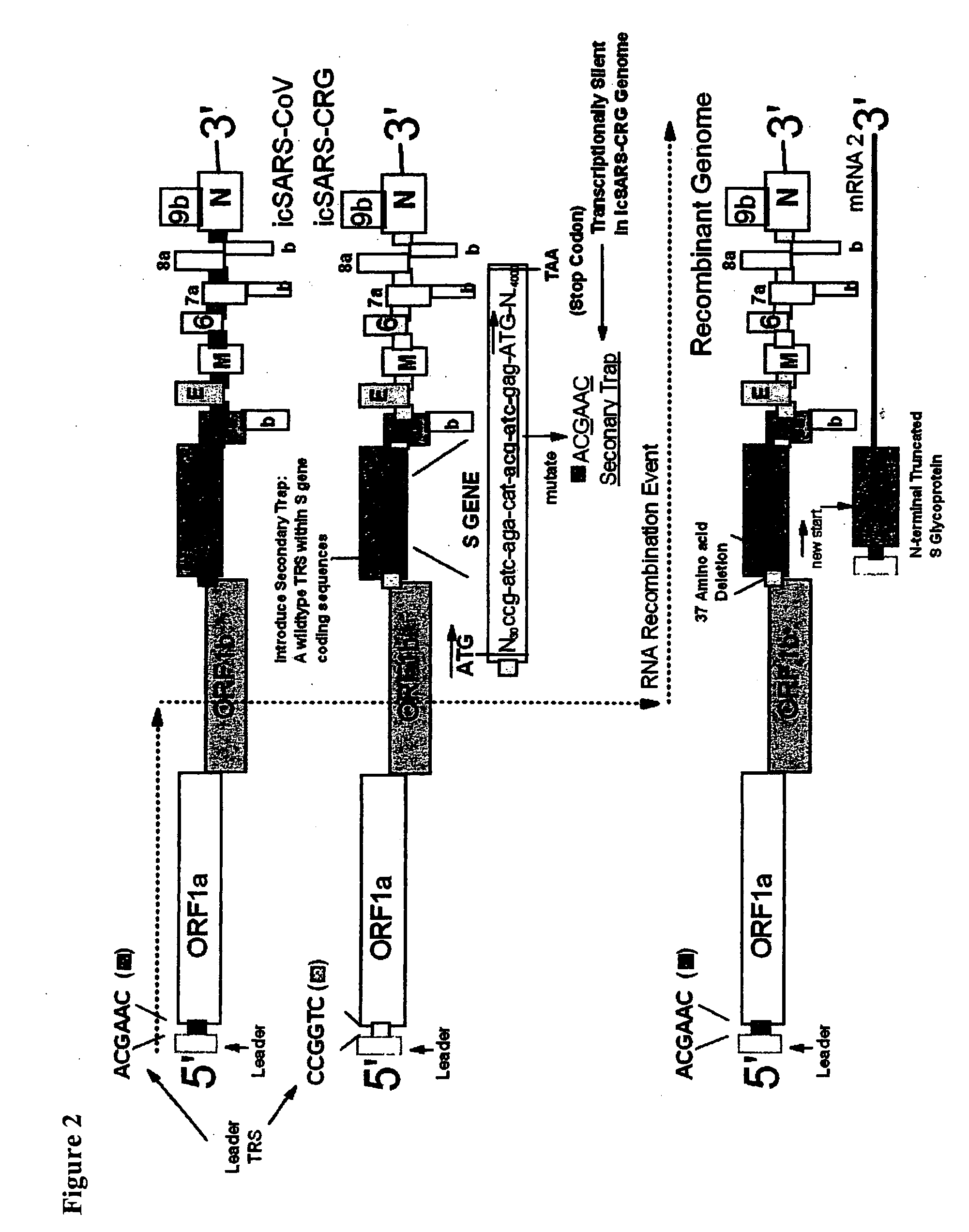
InactiveUS20060240530A1Avoid infectionSsRNA viruses positive-senseVirus peptidesSARS coronavirusViral vector
The present invention provides a cDNA of a severe acute respiratory syndrome (SARS) coronavirus, recombinant SARS coronavirus vectors, and SARS coronavirus replicon particles. Also provided are methods of making the compositions of this invention and methods of using the compositions as immunogens and / or vaccines and / or to express heterologous nucleic acids.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
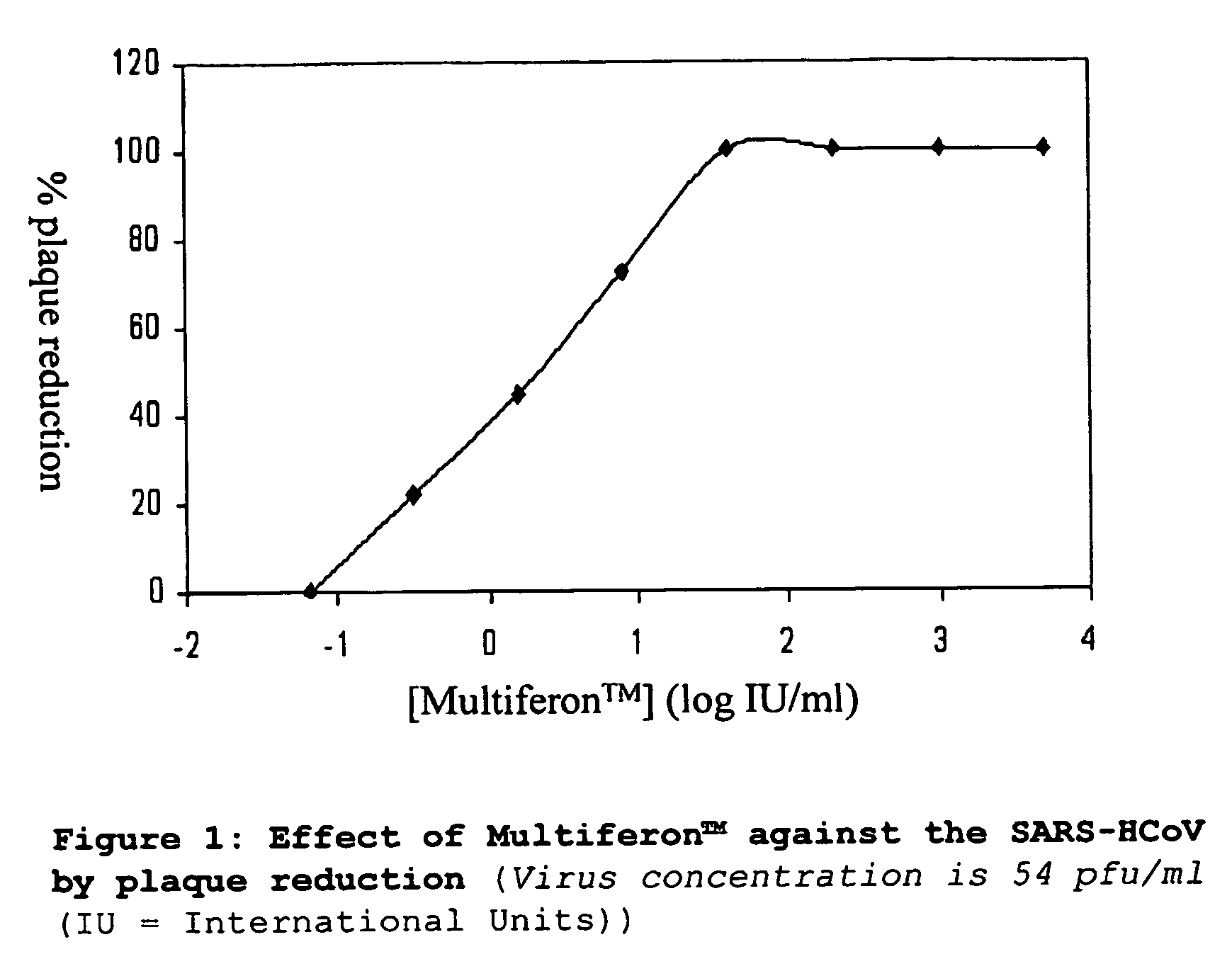
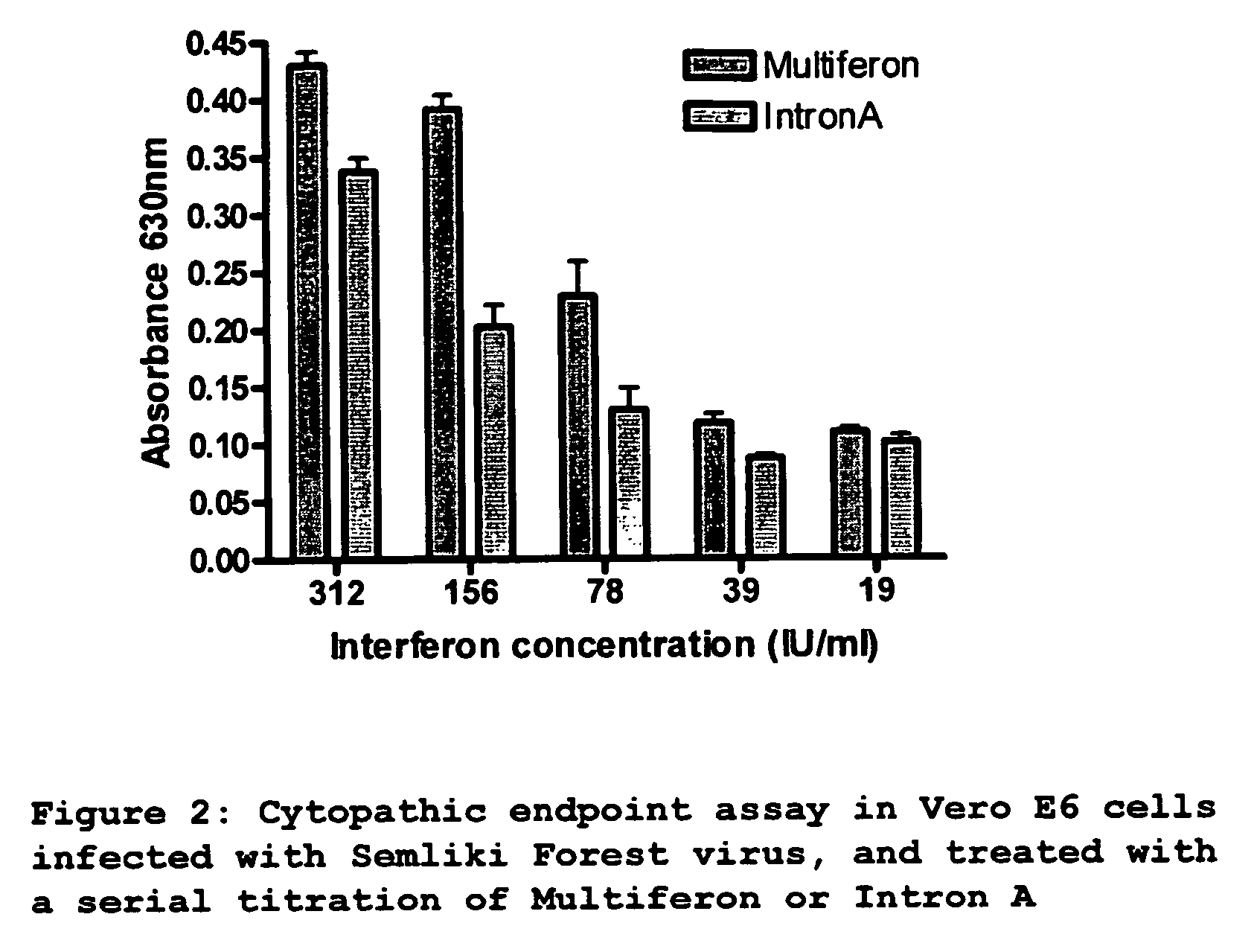
Interferon for treating or preventing a coronaviral infection
InactiveUS20060280723A1Effective treatmentOrganic active ingredientsPeptide/protein ingredientsHuman coronavirusWhite blood cell
The present invention provides a composition and method for use in the prevention or treatment of a coronaviral infection and in particular, the human coronavirus infection termed severe acute respiratory syndrome (SARS) coronavirus (SARS-HCoV). A method of treating a coronaviral infection is provided through the administration of interferon, further the use of interferons in the treatment of a coronaviral infection is also provided. Preferred forms of interferon for use in the invention are multi-subtype interferon products such as multi-subtype, human alpha-interferon derived from white blood cells commercially available as Multiferon.
Owner:VIRANATIVE
Methods For Inhibiting Viruses By Targeting Cathepsin-L Cleavage Sites In The Viruses' Glycoproteins
The disclosure provides methods and compositions useful for inhibiting virus requiring membrane fusion for viral entry, specifically for inhibiting severe acute respiratory syndrome coronavirus (SARS-CoV), Ebola virus (EBOV), Hendra (HeV) and Nipah (NIV) viruses by targeting Cathepsin-L (CatL) cleavages sites in the viruses' glycoproteins.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
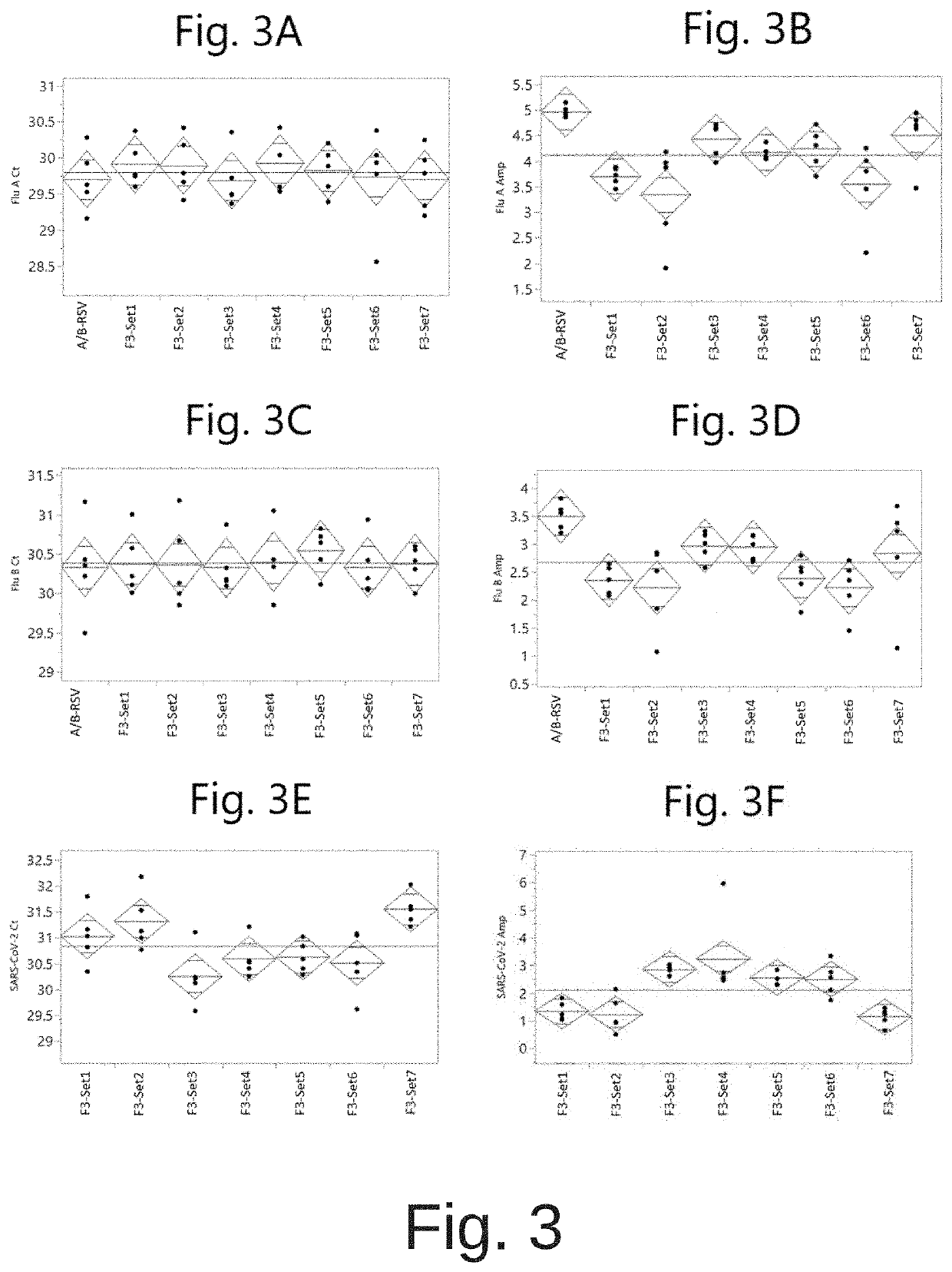
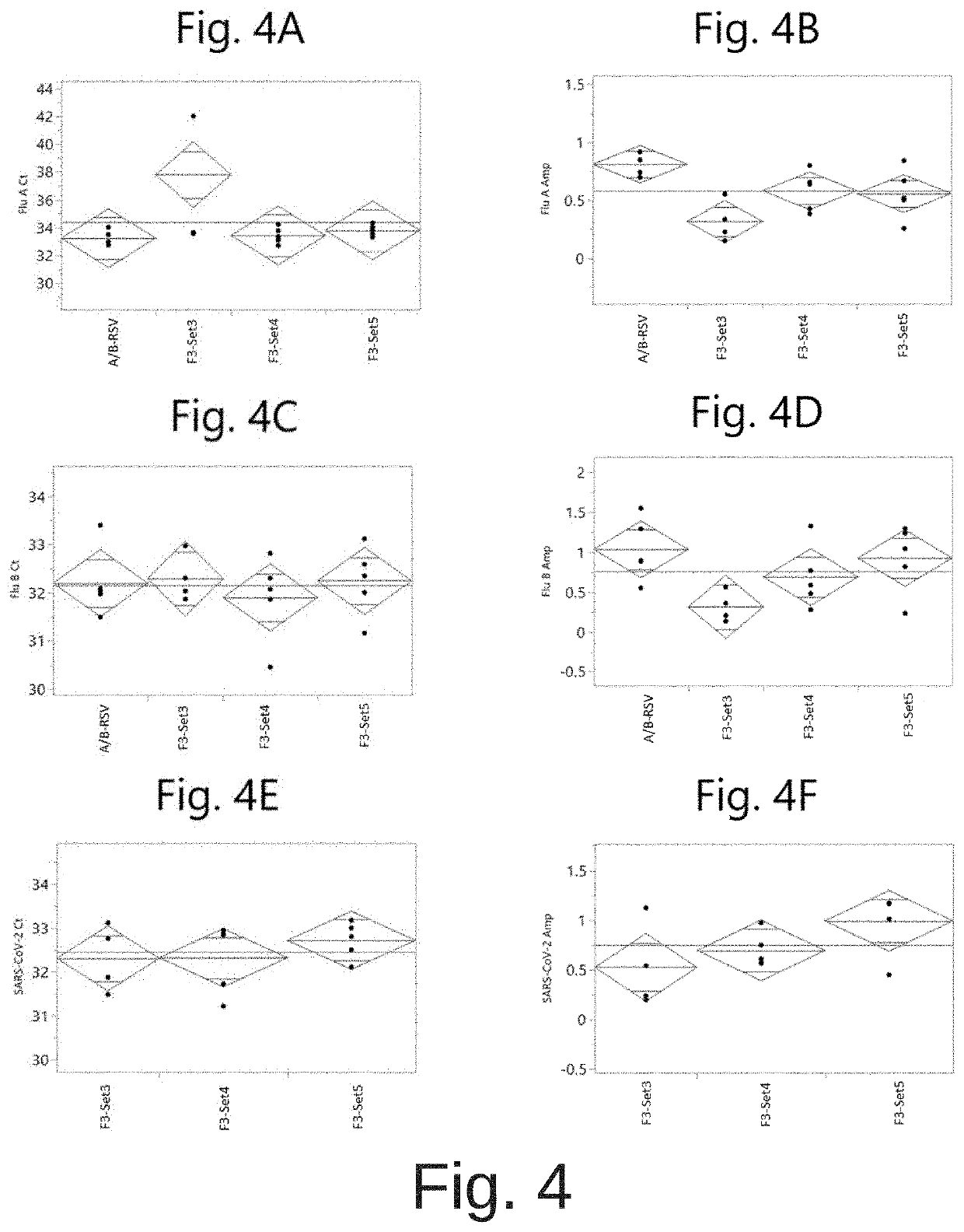
COMPOSITIONS AND METHODS FOR THE SIMULTANEOUS DETECTION OF INFLUENZA A, INFLUENZA B, AND SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2)
PendingUS20220042117A1Enlarge regionMinimizing the potential for cross-contaminationMicrobiological testing/measurementInfluenza A antigenCare setting
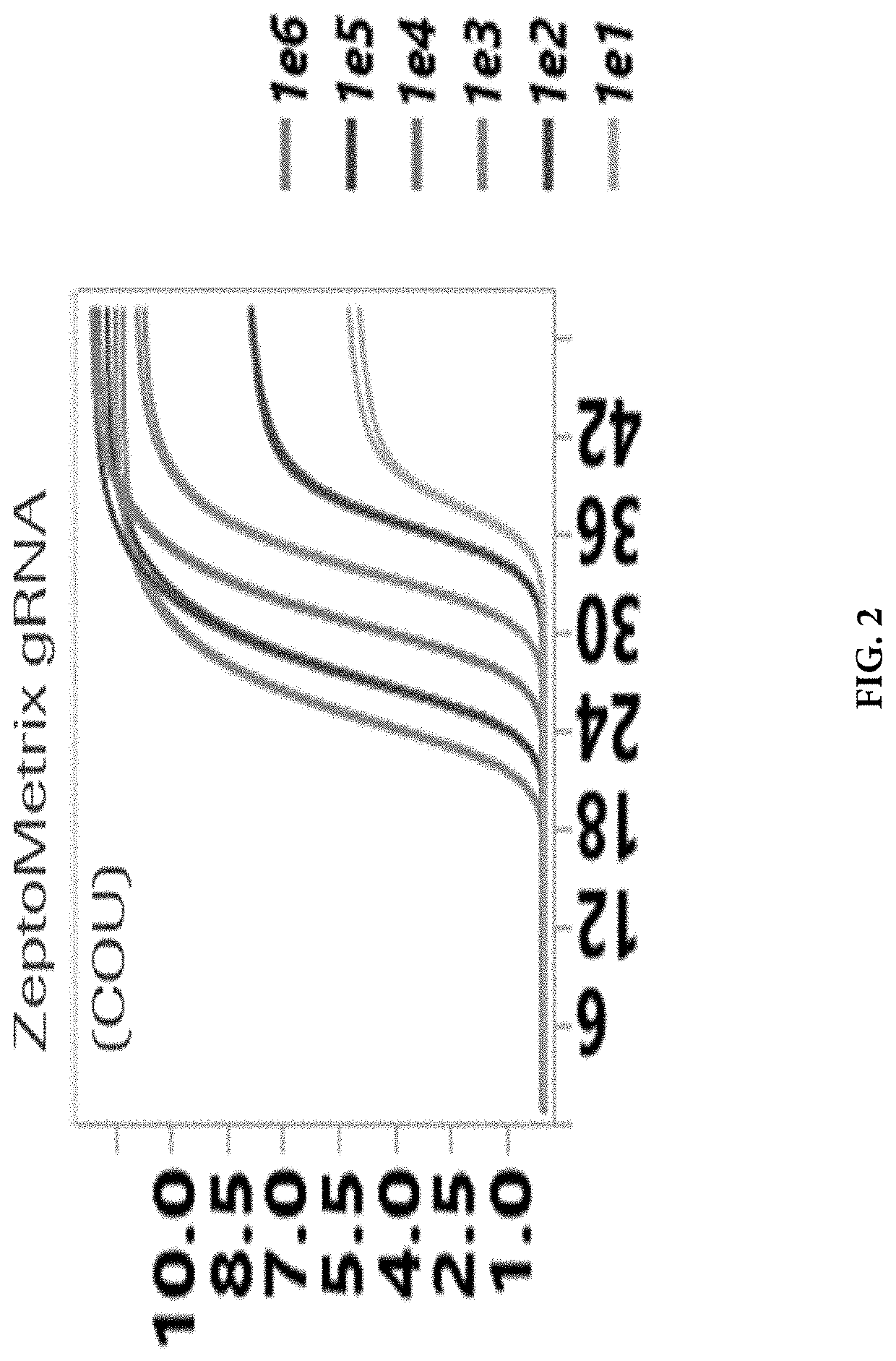
Methods for the rapid detection of the presence or absence of SARS-CoV-2 in biological or non-biological samples are described. These methods are adapted to be performed rapidly in a point-of-care setting. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Specifically, primers and probes targeting SARS-CoV-2 are provided that are designed for the detection of this target. Additionally, kits and reaction vessels containing primers and probes targeting SARS-CoV-2 are provided. Additionally, methods, kits and reaction vessels for the simultaneous rapid detection of the presence or absence of SARS-CoV-2, influenza A, and influenza B in biological or non-biological samples are described.
Owner:ROCHE MOLECULAR SYST INC
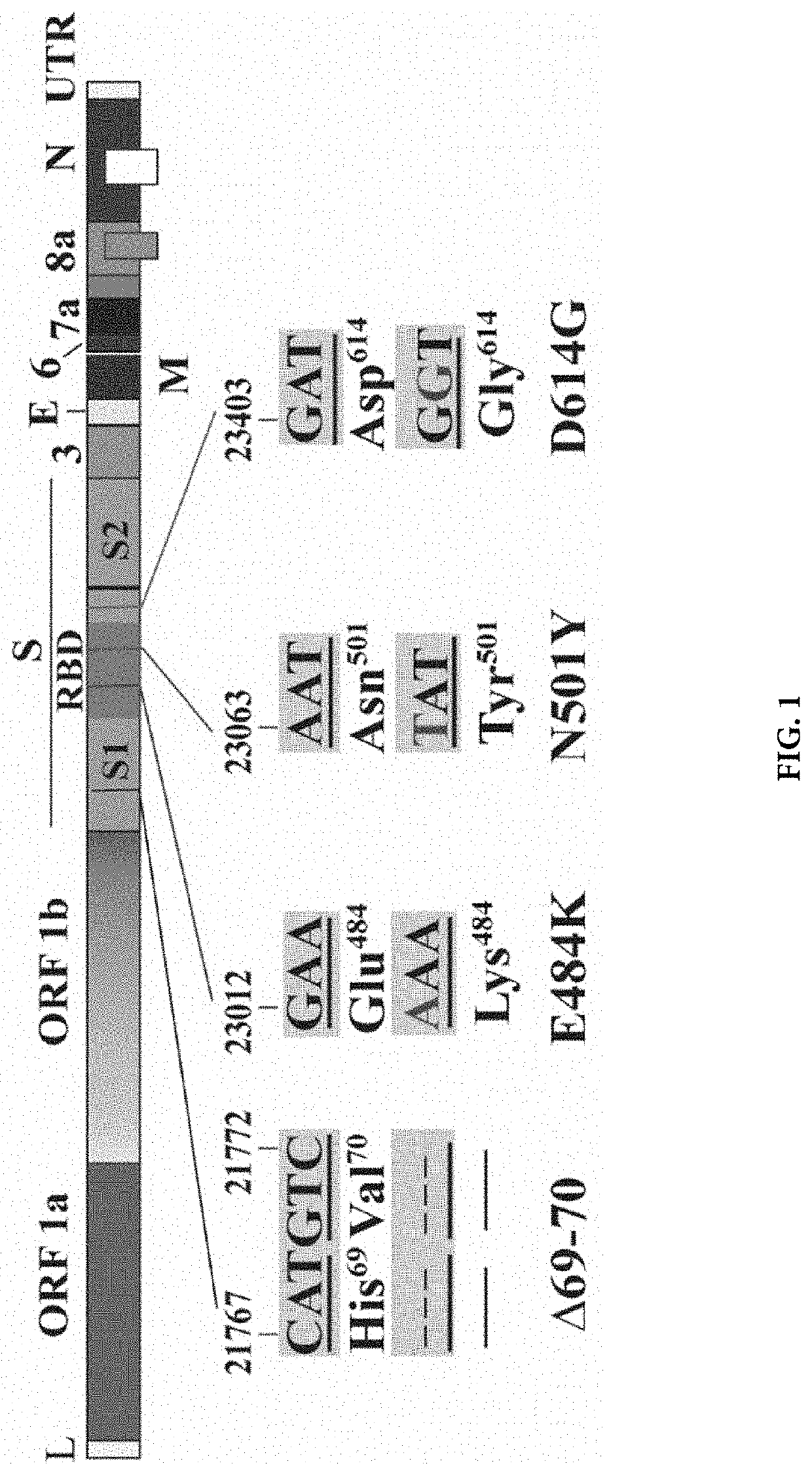
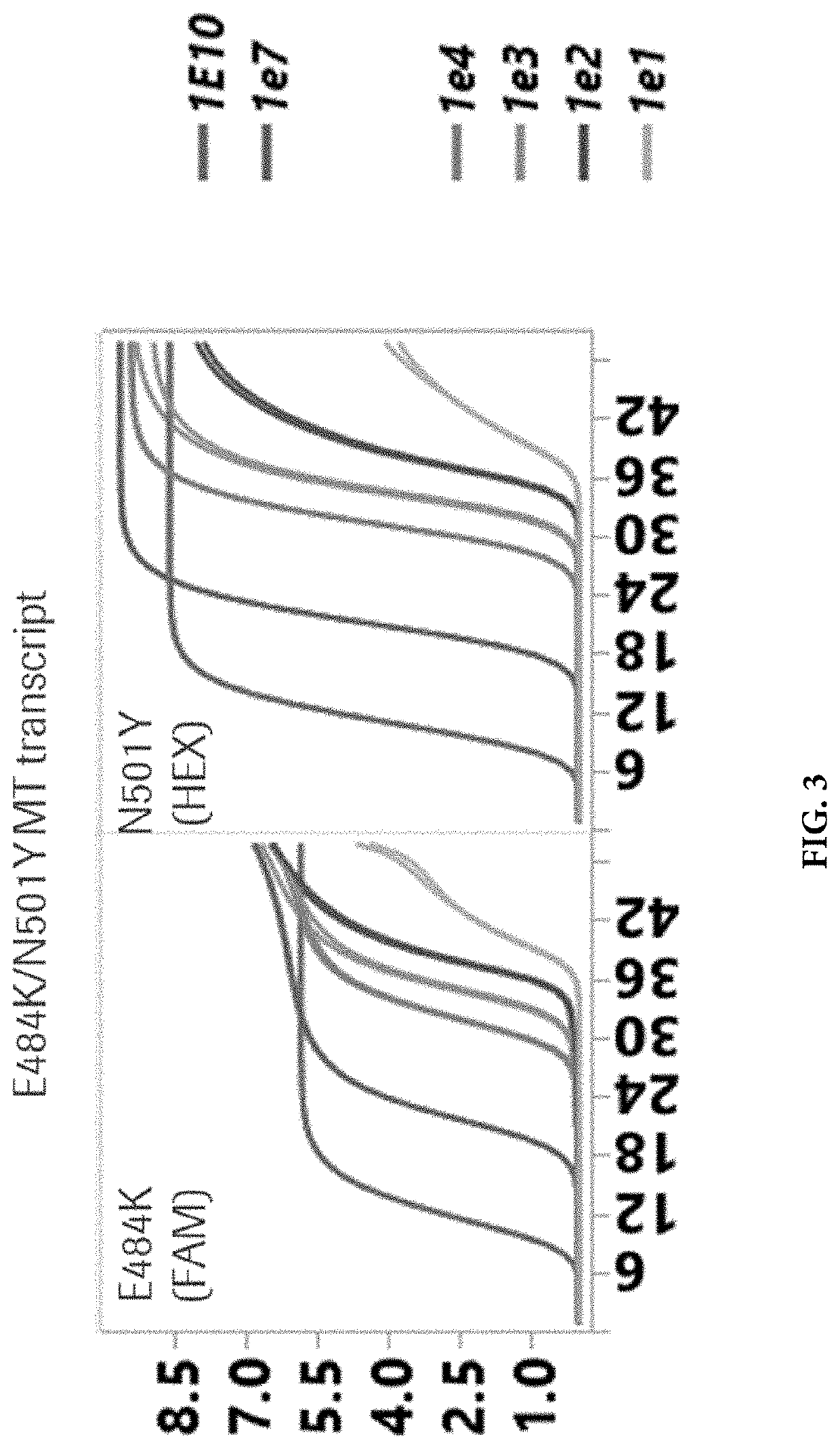
Compositions and methods for detecting severe acute respiratory syndrome coronavirus 2 (sars-cov-2) variants having spike protein mutations
Methods for the rapid detection of the presence of variants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that contain mutations in the Spike (S) protein gene in a biological or non-biological sample are described. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Furthermore, primers and probes targeting SARS-CoV-2 variants containing S gene mutations and kits are provided that are designed for the detection of SARS-CoV-2 variants containing S gene mutations.
Owner:ROCHE MOLECULAR SYST INC
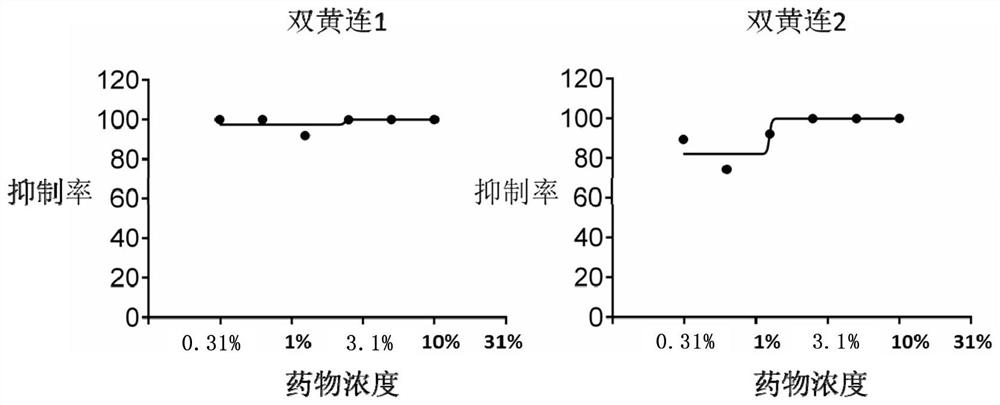
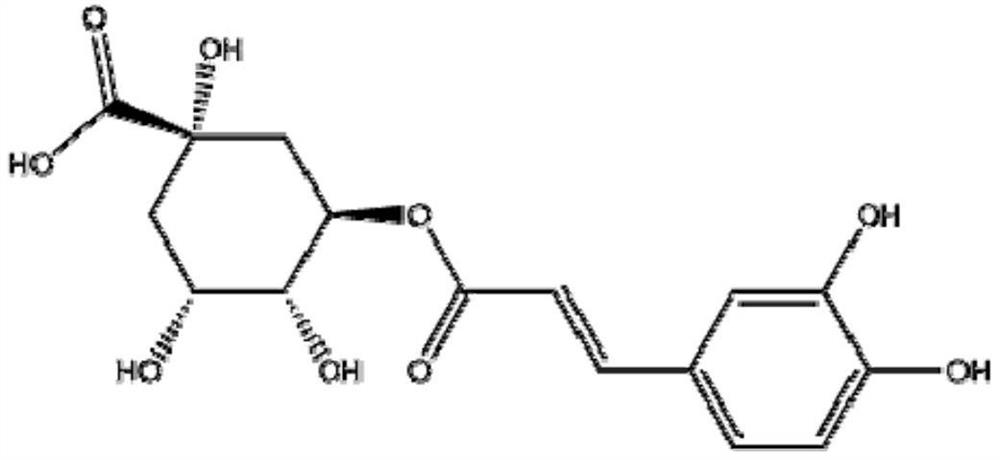
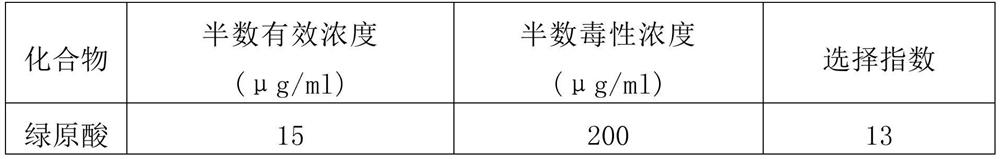
Application of Shuanghuanglian preparation in virus infection resistance
PendingCN113197886AOrganic active ingredientsBiocideMiddle East respiratory syndromeChlorogenic acid
The invention relates to application of a Shuanghuanglian preparation in virus infection resistance. Specifically, the invention provides an application of a chlorogenic acid compound or a pharmaceutically acceptable salt or an extract of the chlorogenic acid compound. Experiments show that the Shuanghuanglian preparation (oral liquid) and the active ingredient chlorogenic acid thereof have obvious antiviral effects on severe acute respiratory syndrome coronavirus (SARS-CoV), influenza virus (influenza virus), Middle East Respiratory Syndrome (MERS) and 2019-nCoV (new coronavirus), and prove that the Chinese patent medicine Shuanghuanglian preparation (oral liquid) can be used for treating virus infectious diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
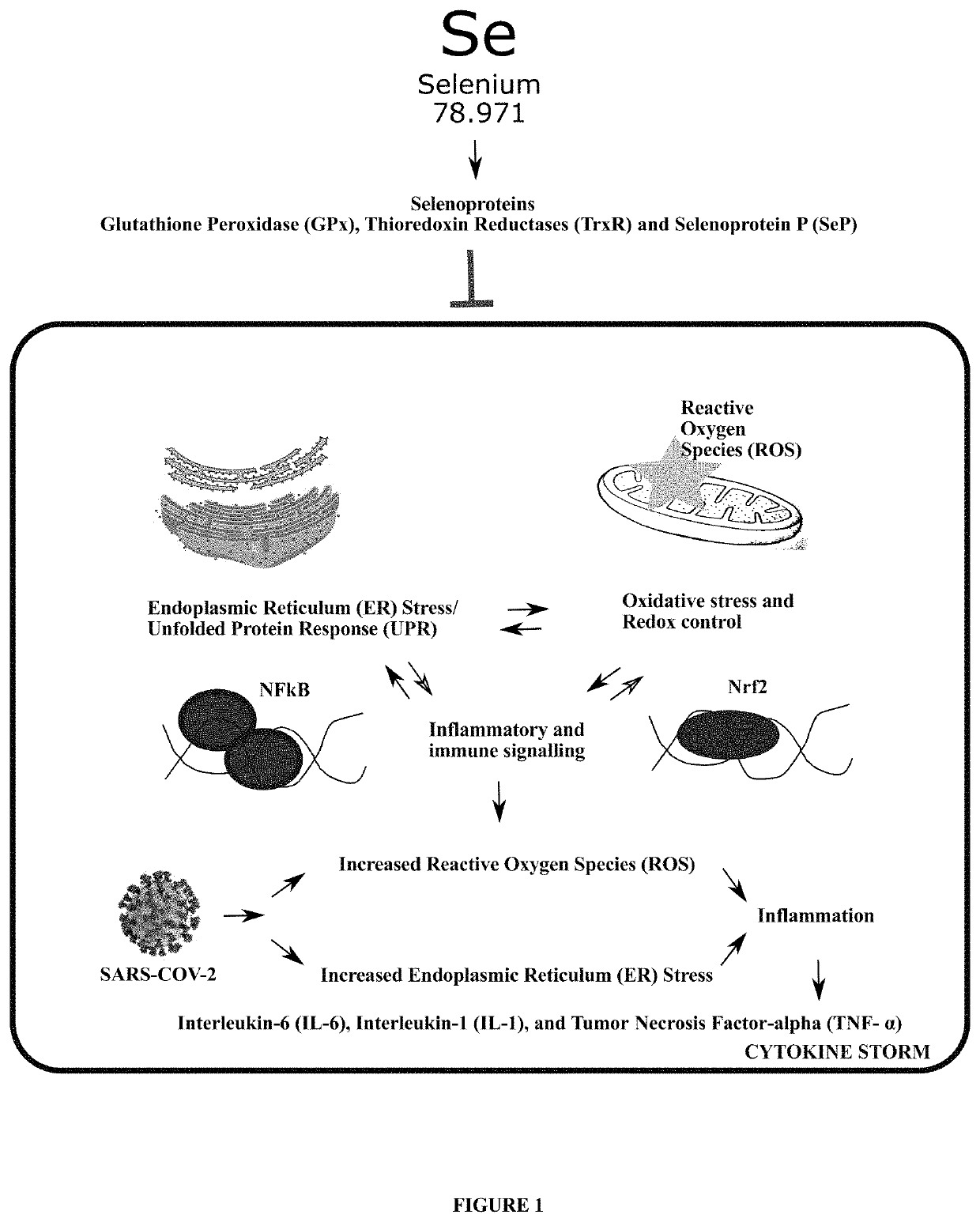
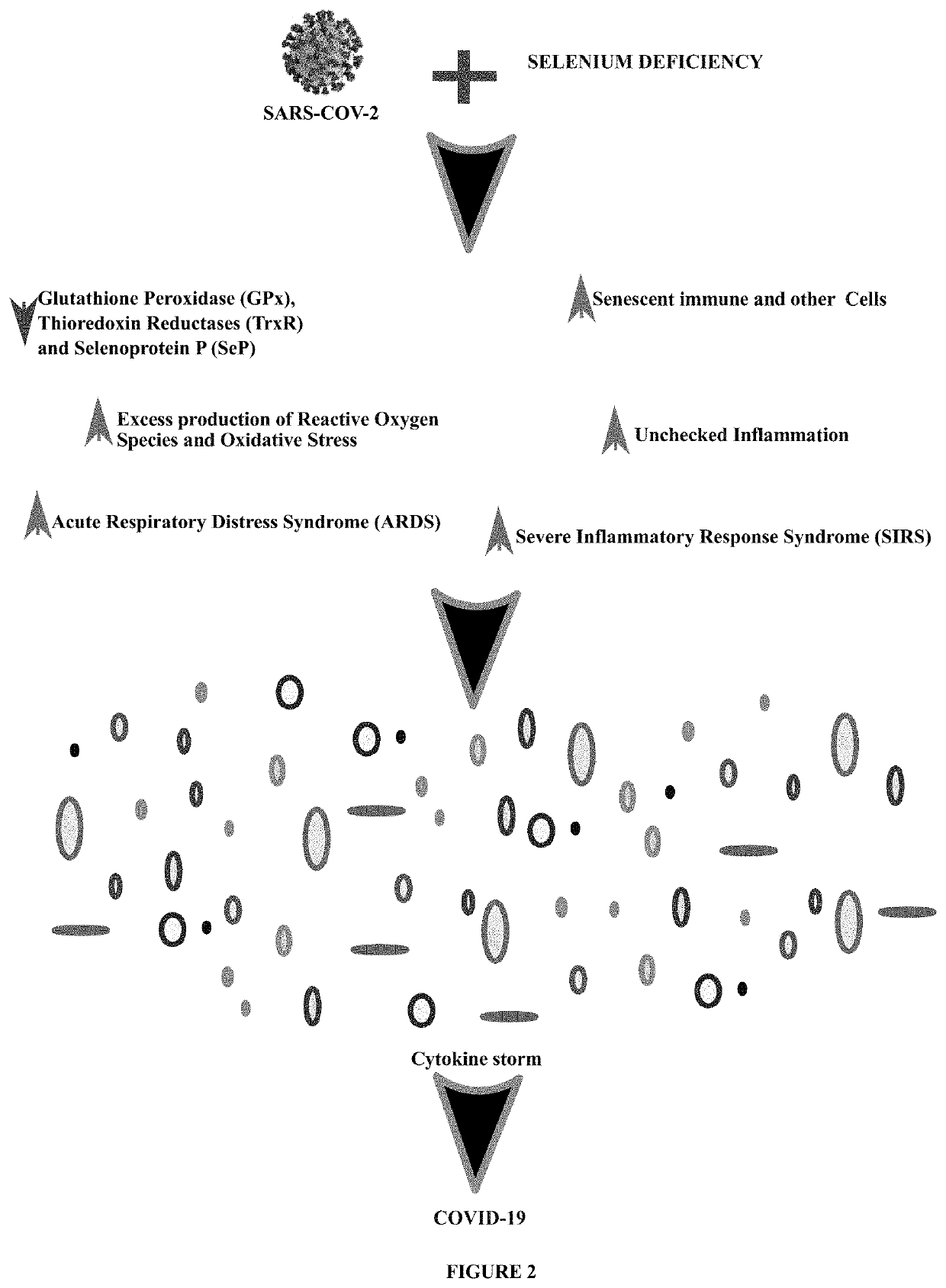
Method of treating and preventing coronavirus disease 19 (covid-19) using a selenium administration
The present invention provides methods for treatment and prevention of Coronavirus Disease 2019 (COVID-19) and any clinical presentations associated with it including Acute Respiratory Distress Syndrome (ARDS), Severe Inflammatory Response Syndrome (SIRS), and / or any state corresponding to a severe acute attack of an inflammatory pathology causing an exacerbation of cytokine storm associated with unregulated oxidative, endoplasmic reticulum, and inflammatory stress mediated cytotoxic effects following the infection with the novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) with at least a pharmacologically acceptable trace element, Selenium (Se) containing molecule, or compound, or drug, or injection, or intravenous infusion. This is achieved by administering to a subject, a therapeutically effective amount of at least a pharmacologically acceptable molecule containing Se at an initial high dose followed by reduced, continuous dosing in subsequent treatment so as to employ its antioxidant, cytokine-modulating, antiviral, immune-enhancing, anti-apoptotic, and anticoagulant properties for the treatment and prevention of COVID-19.
Owner:GHOWEBA MOHAMED SAMIR ELSAYED
Use of angiotensin II type 2 receptor agonist
ActiveUS11123329B1Less side effectsToxic reductionPeptide/protein ingredientsAntiviralsFibrosisRespiratory virus
There is provided N-butyloxycarbonyl-3-(4-imidazol-1-ylmethylphenyl)-5-isobutylthiophene-2-sulfonamide, or a pharmaceutically-acceptable salt thereof, for use in a method of treatment of respiratory virus-induced tissue damage. Such damage may be caused by coronaviruses, including severe acute respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus. N-Butyloxycarbonyl-3-(4-imidazol-1-ylmethylphenyl)-5-iso-butylthiophene-2-sulfonamide alleviate symptoms of diseases caused by those viruses (including coronavirus disease 2019 or COVID-19), such as cough, dyspnea, pneumonia, respiratory distress, respiratory failure and / or fibrosis of organs such as the lungs, the heart or the kidneys, and may thus prevent respiratory virus-induced morbidity and / or mortality. In particular, it has been found in a clinical study that the proportion of patients with COVID-19 needing oxygen treatment was significantly lower for patients that were administered N-butyloxycarbonyl-3-(4-imidazol-1-ylmethylphenyl)-5-iso-butylthiophene-2-sulfonamide compared to placebo.
Owner:VICORE PHARMA AB
Method of identifying severe acute respiratory syndrome corona virus 2 (sars-cov-2) ribonucleic acid (RNA)
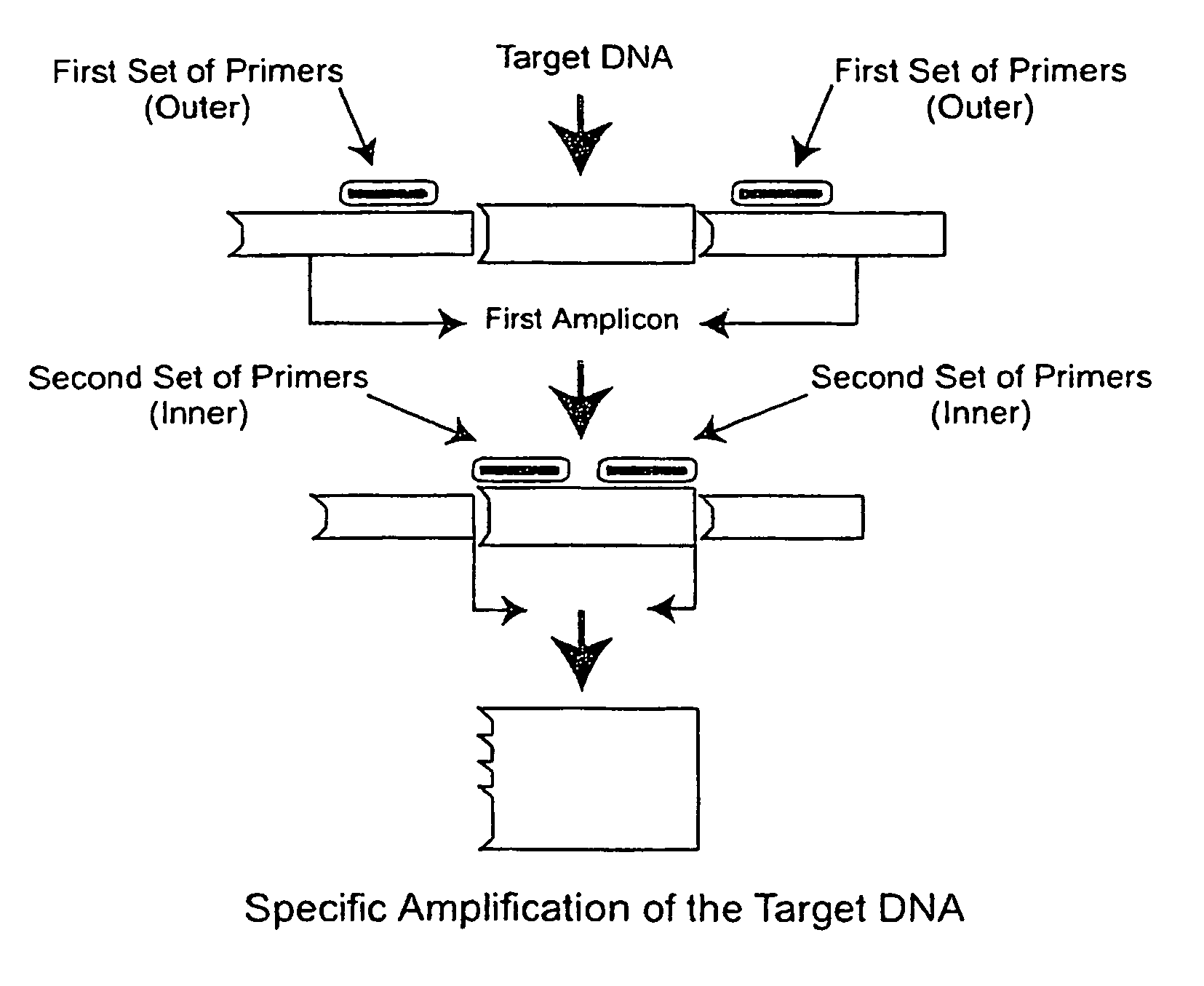
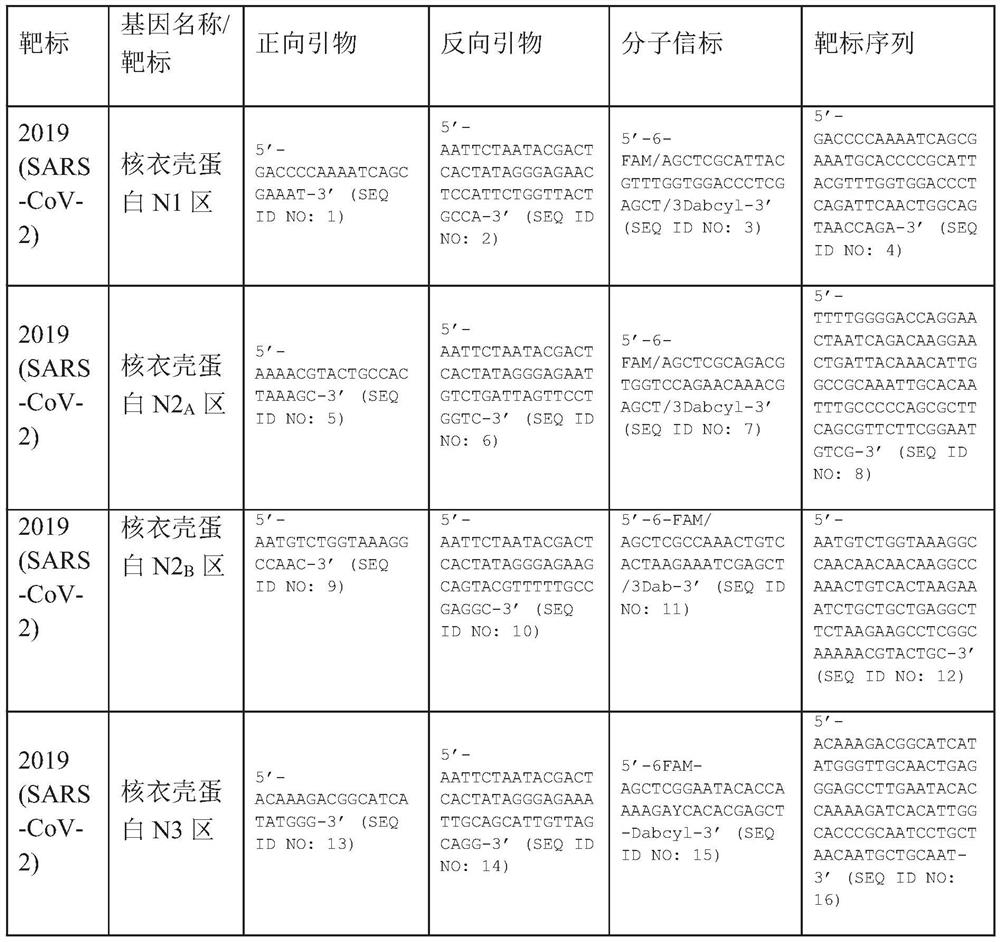
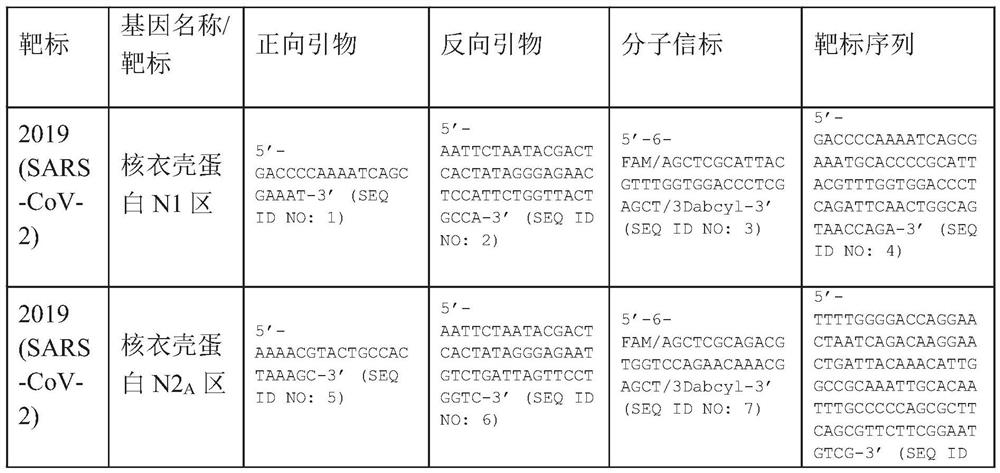
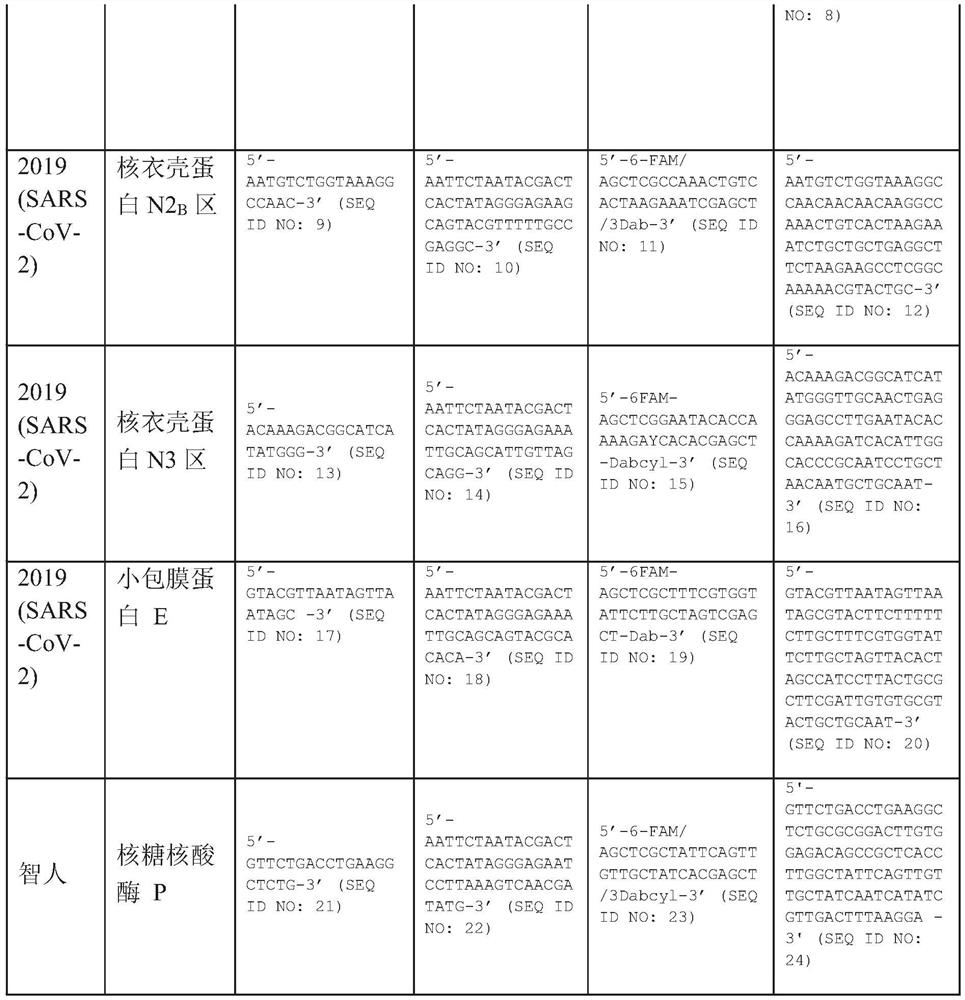
A method for detecting the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a biological sample includes the steps of lysing the biological sample to form a lysate and generating an amplified lysate by performing a nucleic acid sequence-based (NASBA) amplification for a target nucleic acid sequence in the lysate in the presence of: a forward primer having the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13, and SEQ ID NO: 17; a reverse primer having the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14, and SEQ ID NO: 18; and a molecular beacon having the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19 and a fluorophore. The amplified lysate is exposed to an excitation source. A fluorescence of the fluorophore is detected in the amplified lysate exposed to the excitation source The SARS-CoV-2 is determined to be present in the biological sample in response to detecting the fluorescence of the fluorophore.
Owner:TELEFLEX MEDICAL INC
Prodrugs of 1'-substituted carba-nucleoside analogues for antiviral treatment
The present invention provides novel compounds and pharmaceutically acceptable salts or esters thereof. For example, the compound has the structure of Formula V. Also provided is a pharmaceutical composition comprising the compound or a pharmaceutically acceptable salt or ester thereof and a pharmaceutically acceptable carrier. Further provided a method for inhibiting a polymerase of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (SARS-CoV-2 polymerase) or treating viral infection in a subject in need thereof, comprising administering an effective amount of the pharmaceutical composition to the subject, for example, orally.
Owner:COPYCAT SCI INC
New use of angiotensin ii type 2 receptor agonist
There is provided N-butyloxycarbonyl-3-(4-imidazol-1-ylmethylphenyl)-5-isobutylthiophene-2-sulfonamide, or a pharmaceutically-acceptable salt thereof, for use in a method of treatment of respiratory virus-induced tissue damage. Such damage may be caused by coronaviruses, including severe acute respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus. N-Butyloxycarbonyl-3-(4-imidazol-1-ylmethylphenyl)-5-iso-butylthiophene-2-sulfonamide alleviate symptoms of diseases caused by those viruses (including coronavirus disease 2019 or COVID-19), such as cough, dyspnea, pneumonia, respiratory distress, respiratory failure and / or fibrosis of organs such as the lungs, the heart or the kidneys, and may thus prevent respiratory virus-induced morbidity and / or mortality. In particular, it has been found in a clinical study that the proportion of patients with COVID-19 needing oxygen treatment was significantly lower for patients that were administered N-butyloxycarbonyl-3-(4-imidazol-1-ylmethylphenyl)-5-iso-butylthiophene-2-sulfonamide compared to placebo.
Owner:VICORE PHARMA AB
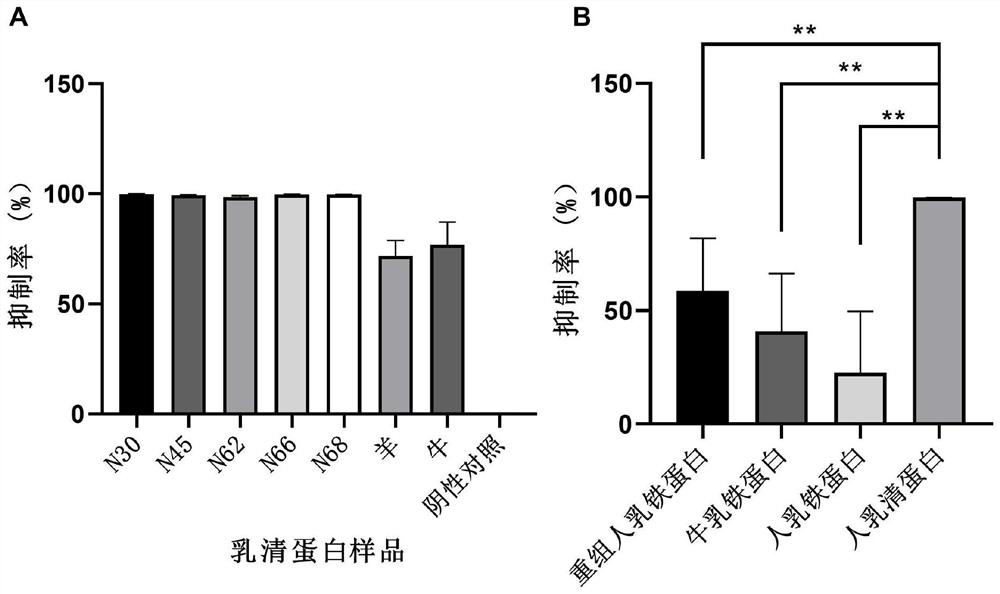
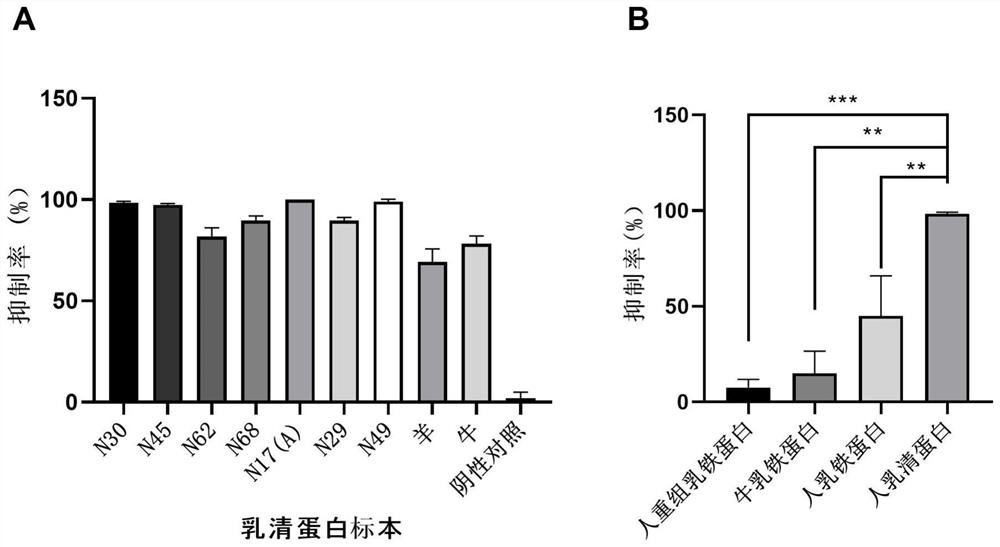
Application of whey protein as broad-spectrum inhibitor of coronaviruses
PendingCN113855787AAvoid stickingInhibit entryPeptide/protein ingredientsAntiviralsDiseaseWhey protein
The invention provides novel application of whey protein and degraded products thereof. The whey protein and the degraded products thereof can be used for preventing and / or treating diseases caused by coronaviruses such as serious acute respiratory syndrome coronavirus (SARS-CoV), novel coronavirus (SARS-CoV-2) and similar novel coronaviruses such as pangolin coronavirus (xCoV) as broad-spectrum inhibitors of the coronaviruses. The whey protein can be used for inhibiting adhesion, entry and replication of viruses, is an effective multi-target inhibitor and is high in safety, so that the whey protein can be applied to prevention and / or treatment of the diseases caused by the coronaviruses.
Owner:PEKING UNIV +2
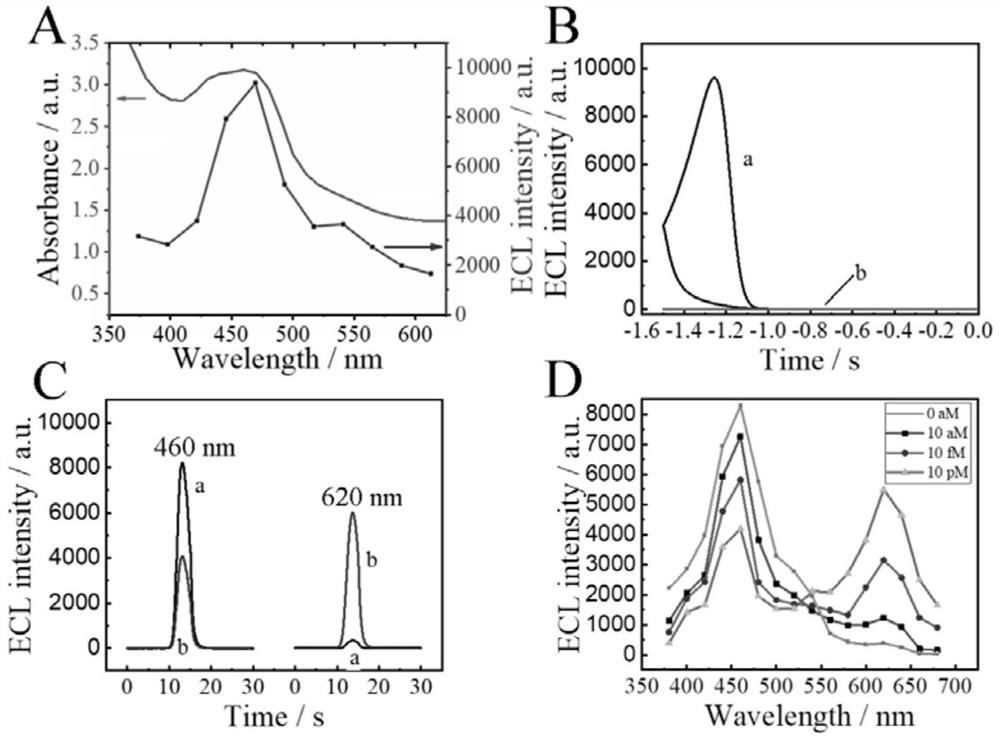
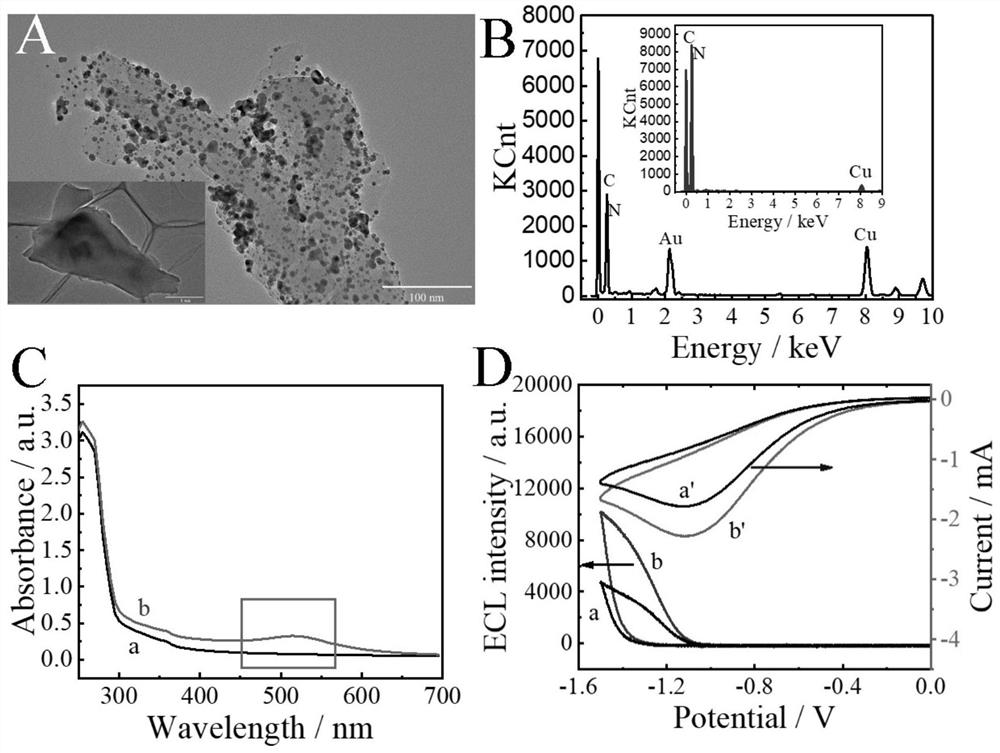
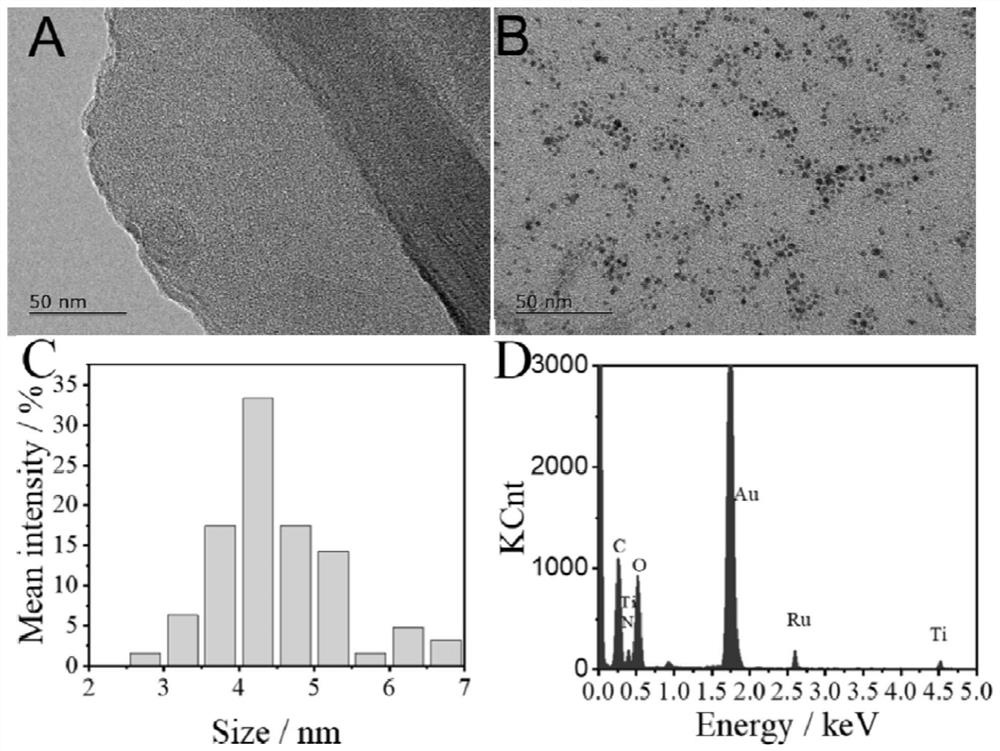
Ratio-type ECL biosensor as well as preparation method and application thereof
ActiveCN113278683AGood receptorMicrobiological testing/measurementAgainst vector-borne diseasesA-DNAElectrochemiluminescence
The invention constructs a ratio electrochemical luminescence (ECL) biosensor, which is used for detecting the RNA-dependent RNA polymerase (RdRp) gene of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). The SARS-COV-2RdRp gene participates in the entropy-driven cyclic amplification reaction, then a piece of Bandage DNA is output, and can be combined with the other two single chains S1 and S2 to form a double-chain DNAWalker, and the next cyclic reaction is started. After the walking process of the double-foot DNA Walker is completed, a hairpin structure at the top of a DNA tetrahedron (TDNAs) is removed. Then, a PEI-Ru@Ti3C2@AuNPs-S7 probe is used for being specifically combined with a TDNAs hairpin part cut off from the surface of Au-g-C3N4, and signal change is achieved through ECL-RET (electrochemiluminescence resonance energy transfer).
Owner:JIANGSU INST OF NUCLEAR MEDICINE
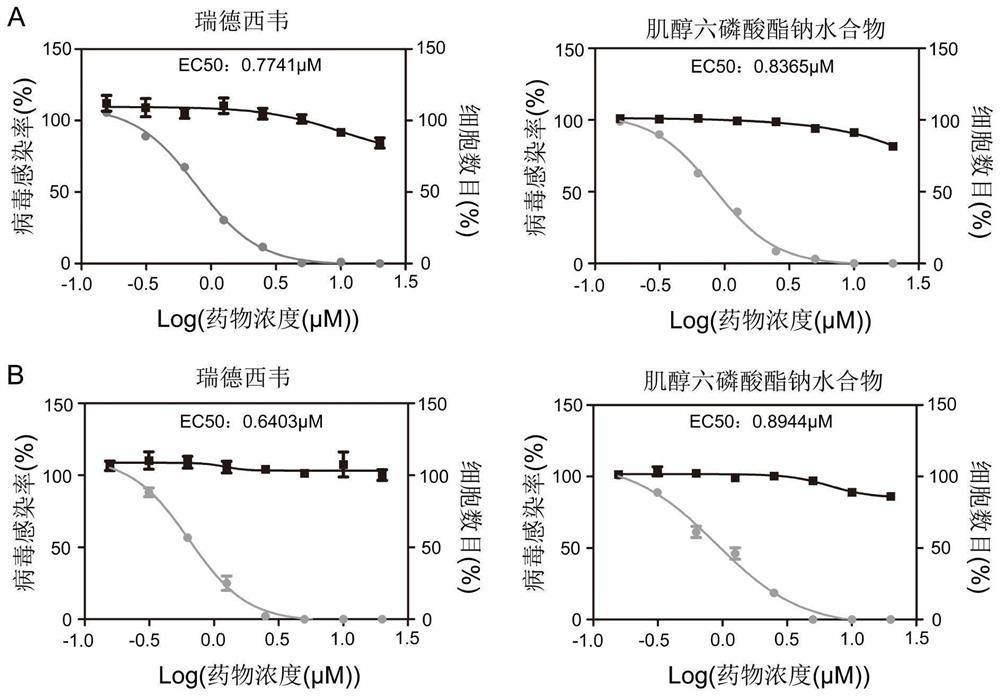
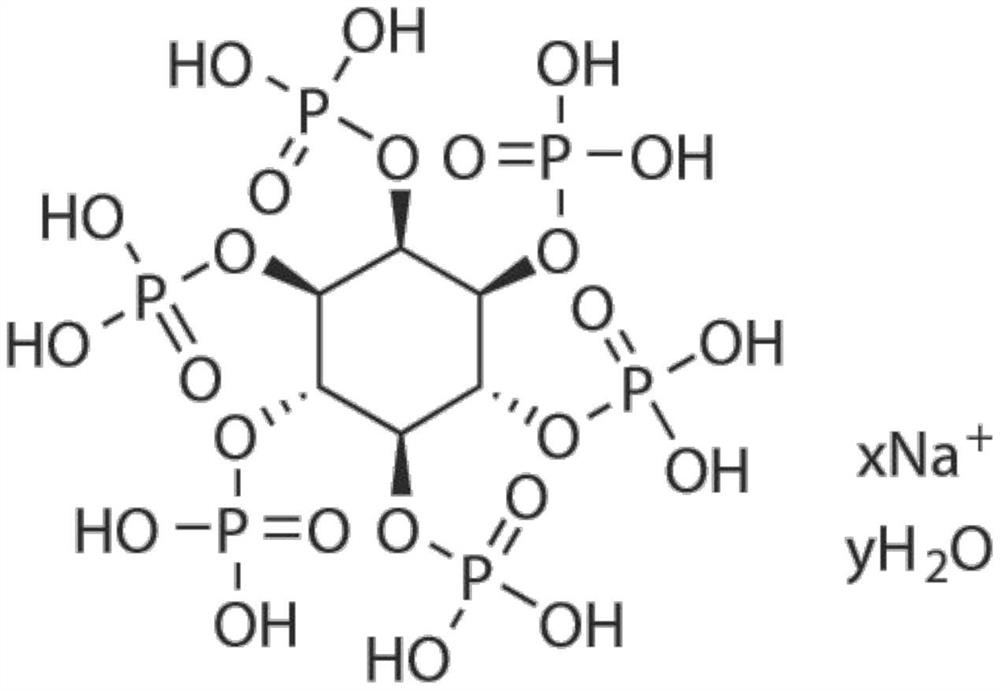
Application of small molecule compound sodium phytate hydrate in preparation of medicine for resisting SARS-CoV-2
ActiveCN113712976ALow toxicityAvoid infectionOrganic active ingredientsAntiviralsGreen monkeyBULK ACTIVE INGREDIENT
The invention discloses application of a small molecule compound sodium phytate hydrate in preparation of a medicine for resisting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); the medicine for resisting the SARS-CoV-2 is a pharmaceutical composition which takes the sodium phytate hydrate as a unique active ingredient or contains the sodium phytate hydrate; and the medicine for resisting the SARS-CoV-2 is a medicine for preventing or treating SARS-CoV-2 infection. According to the invention, an SARS-CoV-2 susceptible cell line, including African green monkey kidney cells Vero E6 and human lung adenocarcinoma cells Calu-3, is utilized to detect the anti-SARS-CoV-2 activity of the sodium phytate hydrate. Experimental results show that the sodium phytate hydrate can effectively inhibit infection of the SARS-CoV-2 on the susceptible cells, is relatively low in cytotoxicity, is expected to be used as a medicine for effectively resisting SARS-CoV-2 infection, and has an application prospect.
Owner:THE NAVAL MEDICAL UNIV OF PLA
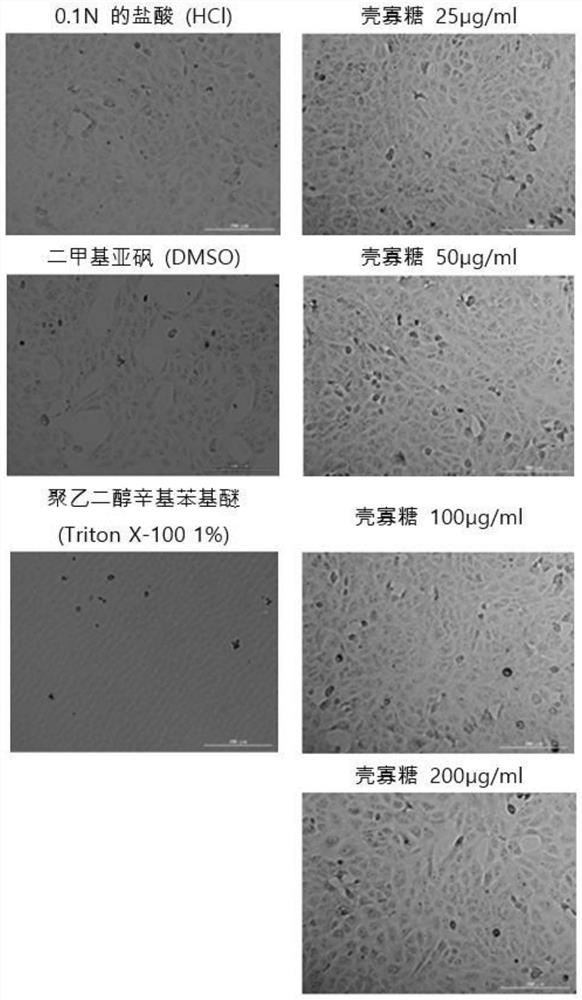
Composition for preventing or treating coronavirus infectious diseases comprising low molecular chitosan
PendingCN114767707AInhibition of translationInhibition of replicationOrganic active ingredientsCosmetic preparationsMolecular biologySevere acute respiratory syndrome coronavirus RNA
The present invention relates to a composition for preventing or treating coronavirus infectious diseases comprising low-molecular chitosan or chitosan oligosaccharide, and more particularly, to a composition comprising low-molecular chitosan or chitosan oligosaccharide for preventing or treating coronavirus infectious diseases comprising low-molecular chitosan or chitosan oligosaccharide for preventing or treating diseases caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The present invention relates to a composition for preventing or treating a coronavirus infection disease, which comprises a low molecular weight chitosan or chitosan oligosaccharide having a specific molecular weight range and exhibiting an antiviral effect (Severate response signal coronavius2), and a method for preparing the composition for preventing or treating a coronavirus infection disease by using the composition, and more specifically, to a composition for preventing or treating a coronavirus infection disease by using a low molecular weight chitosan or chitosan oligosaccharide having a specific molecular weight range and having an antiviral effect. The composition containing the low-molecular chitosan or chitosan oligosaccharide in a specific molecular weight range can inhibit translation, replication and proliferation of the severe acute respiratory syndrome coronavirus 2, and can effectively prevent or treat novel coronavirus pneumonia (COVID-19) caused by infection of the severe acute respiratory syndrome coronavirus 2.
Owner:艾美科健株式会社
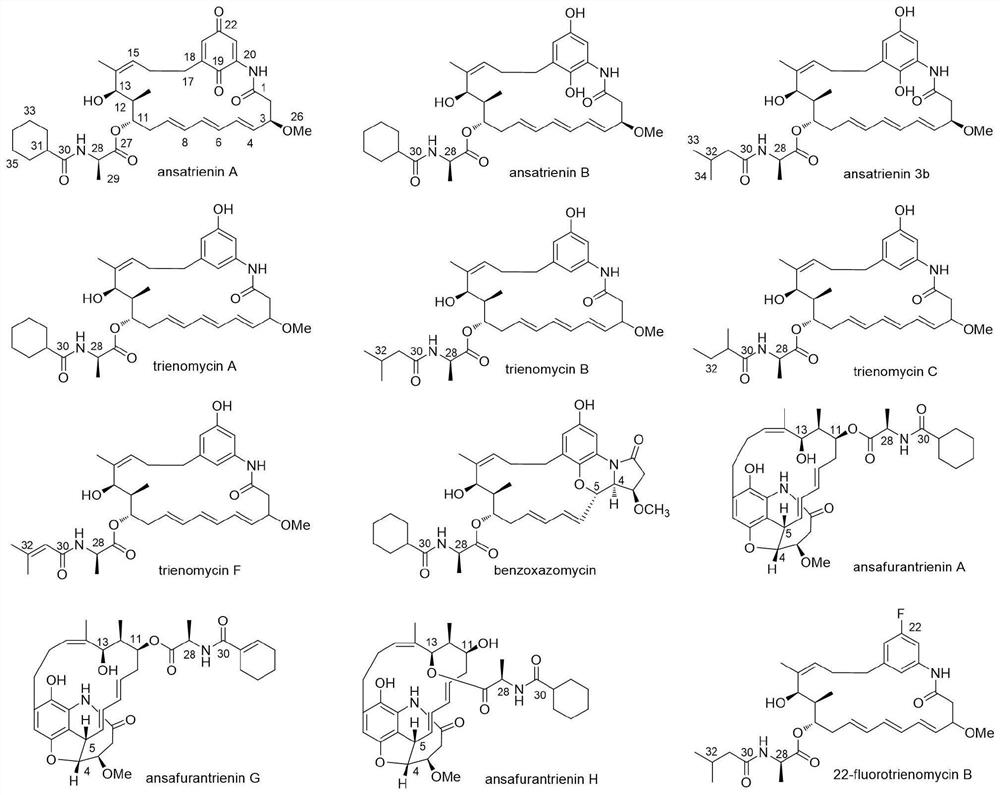
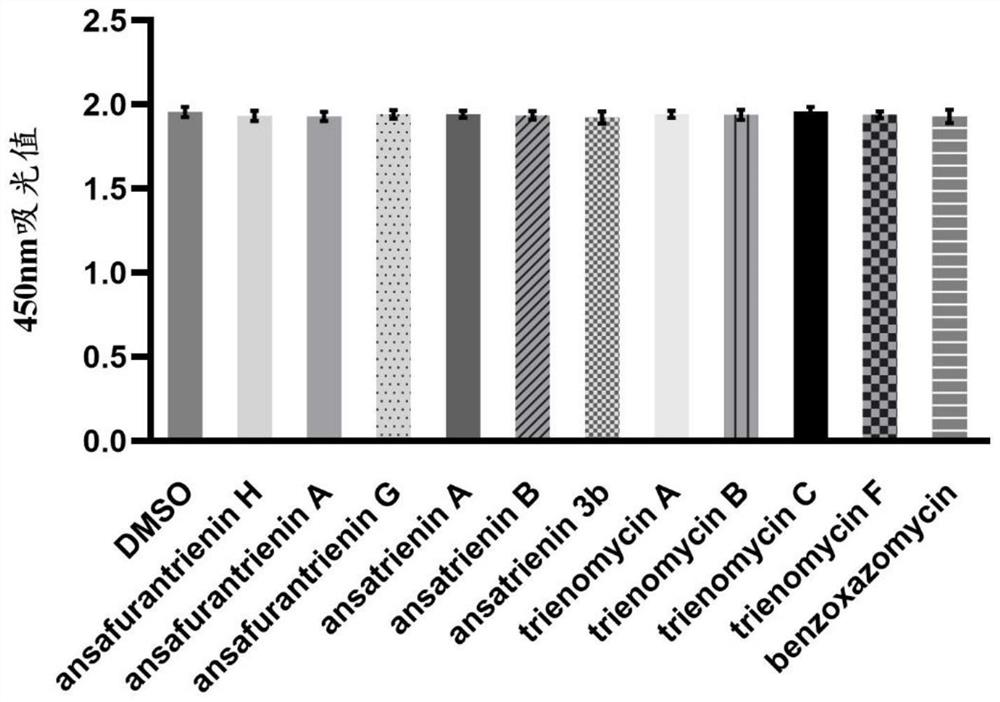
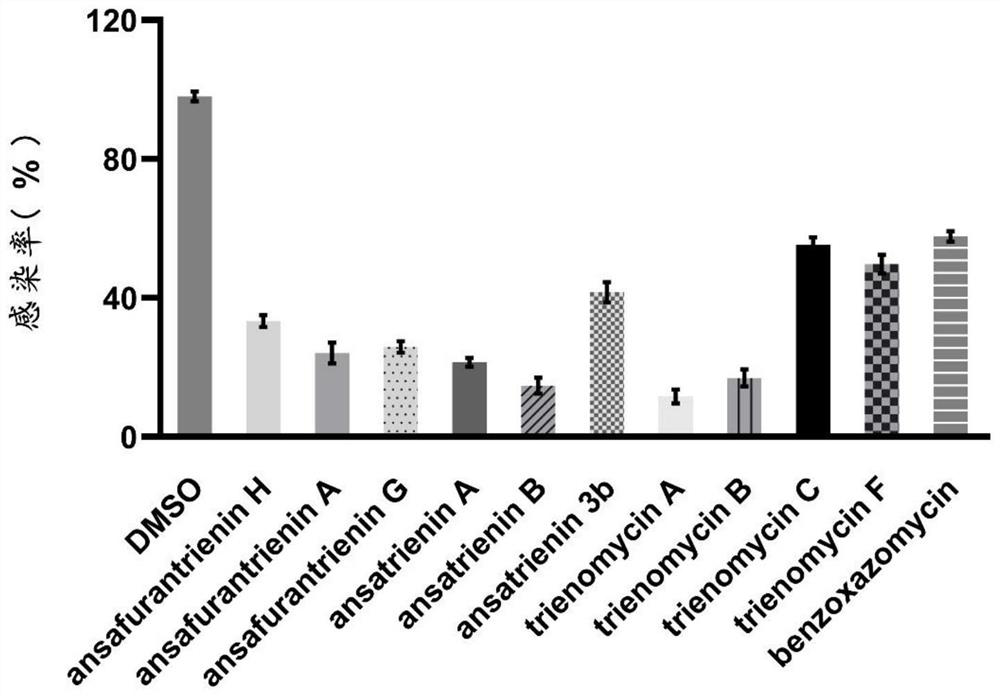
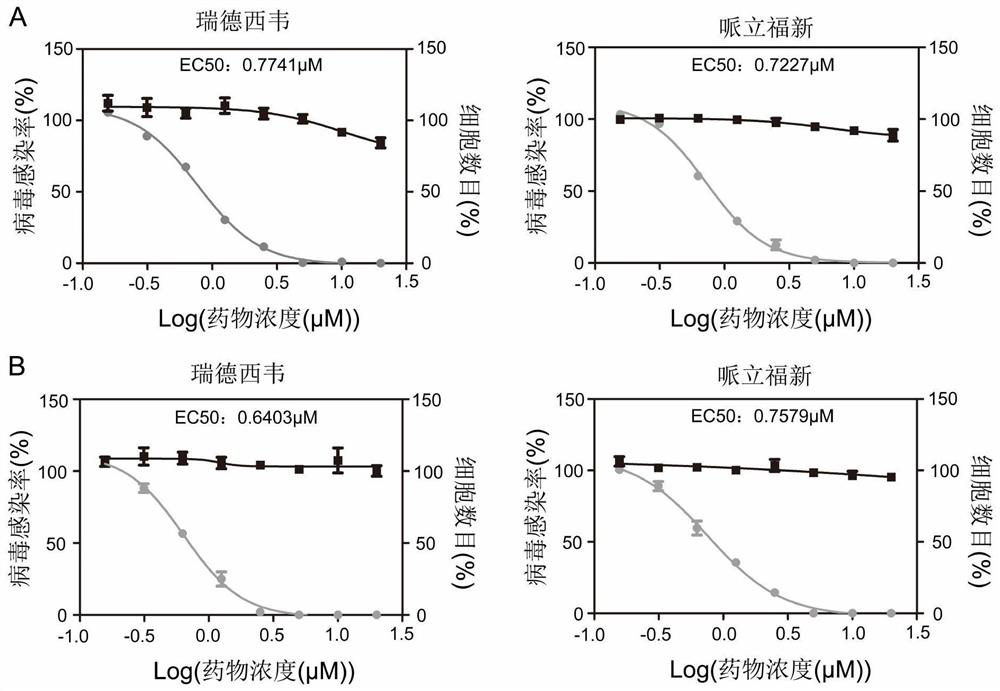
Application of ansatriene compound in antiviral infection medicine and preparation method of ansatriene compound
PendingCN114796204AHas inhibitory effectLow cytotoxicityOrganic active ingredientsAntimycoticsTick-borne encephalitis virus TBEVCoronavirus
The invention relates to the technical field of medicines, and relates to application of an ansatriene compound in preparation of medicines or pharmaceutical compositions for resisting infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), tick-borne encephalitis virus (TBEV), west nile virus (WNV), yellow fever virus (YFV) and chikungunya fever virus (CHIKV) and a preparation method of the ansatriene compound.
Owner:THE NAVAL MEDICAL UNIV OF PLA
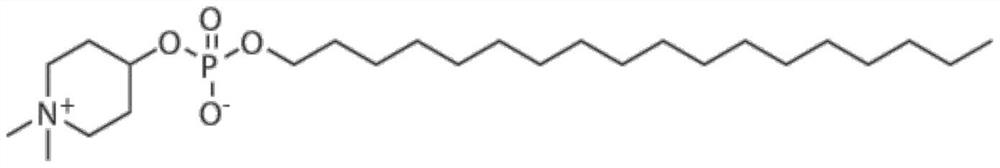
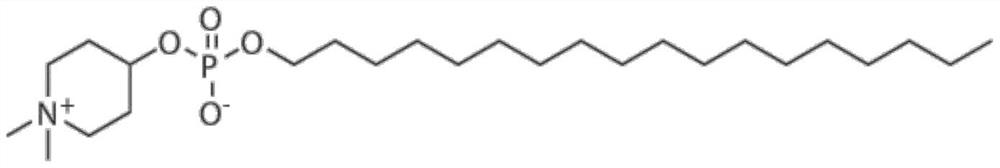
Application of small molecule compound piperifoside in preparation of SARS-CoV-2 resisting medicine
PendingCN114515291ALow toxicityAvoid infectionOrganic active ingredientsAntiviralsCancer cellBULK ACTIVE INGREDIENT
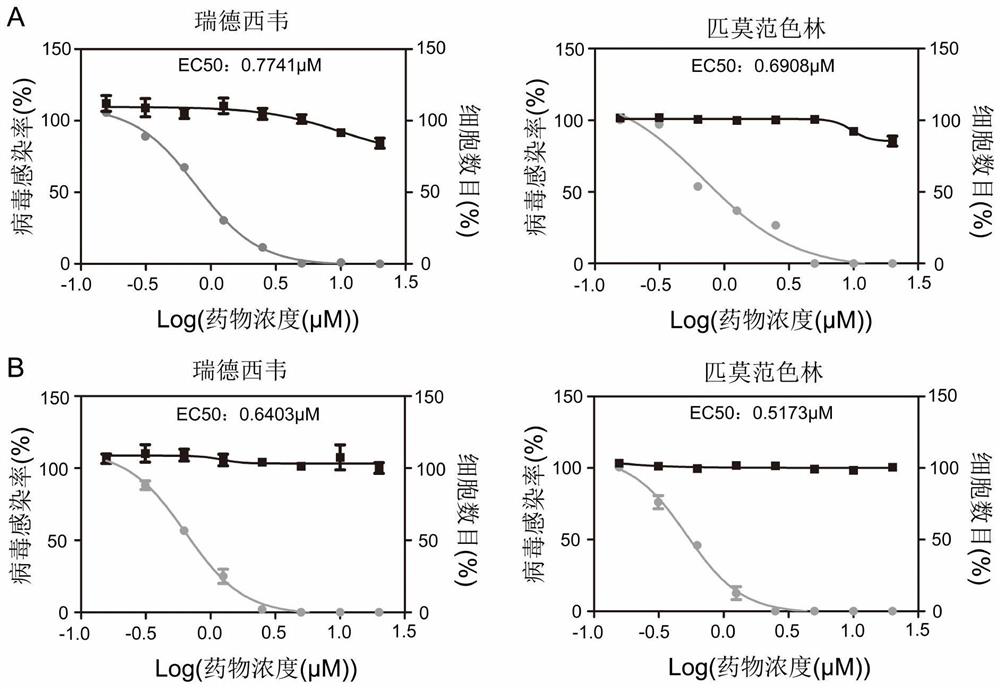
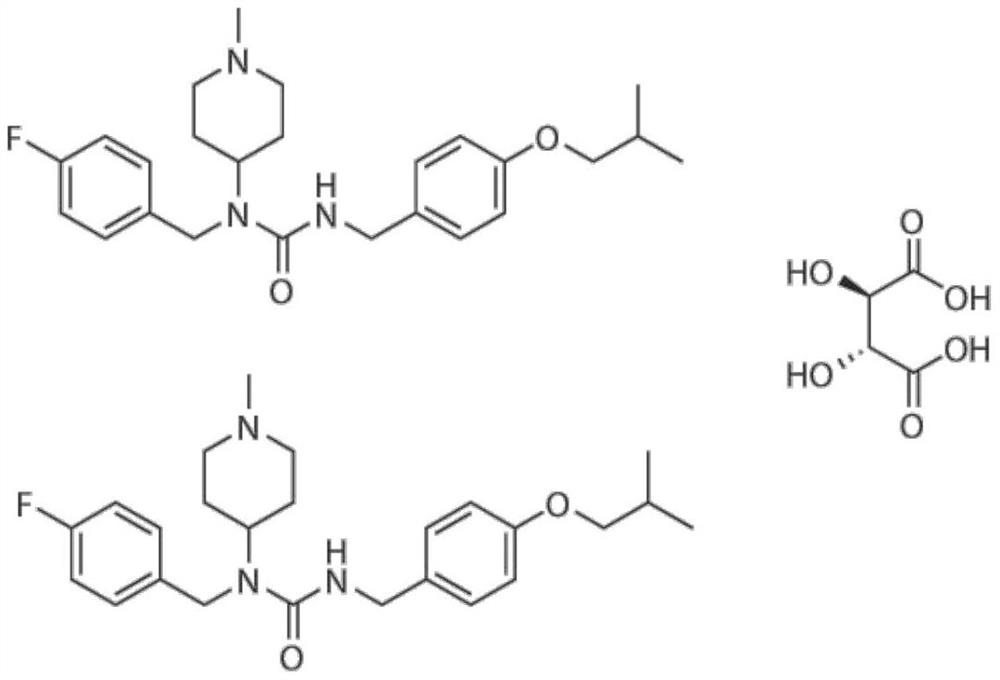
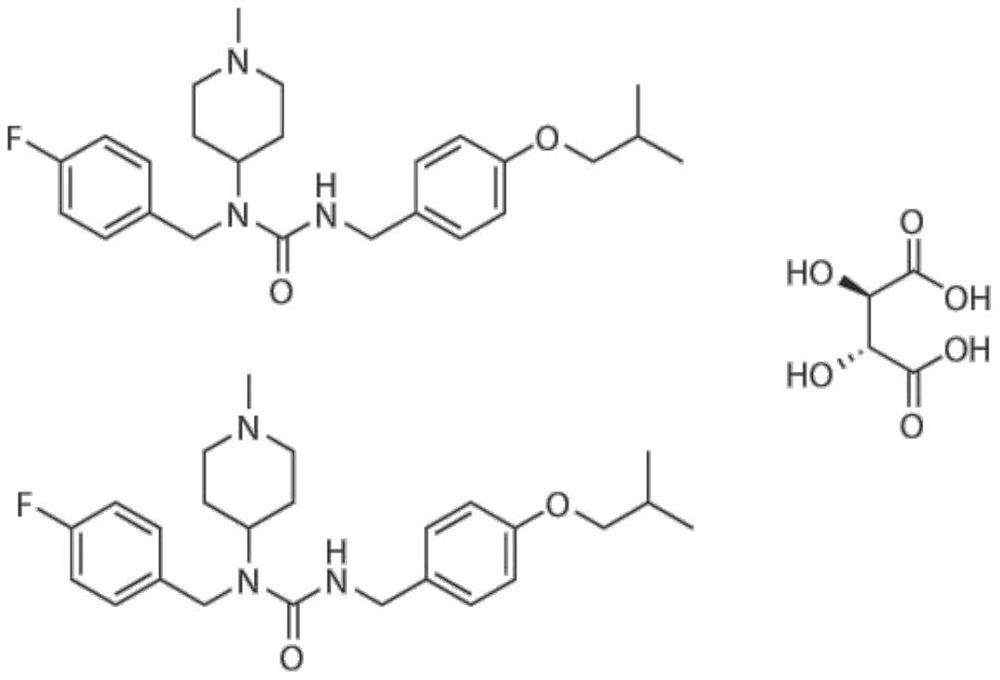
The invention discloses an application of a small molecule compound piperifoside in preparation of a medicine for resisting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the medicine for resisting SARS-CoV-2 is a medicine composition taking piperifoside as a unique active ingredient or containing piperifoside, and the medicine for resisting SARS-CoV-2 refers to a medicine for preventing or treating SARS-CoV-2 infection. According to the invention, a susceptible cell line of SARS-CoV-2, including African green monkey kidney cells Vero E6 and human lung adenocarcinoma cells Clu-3, is utilized to detect the anti-SARS-CoV-2 activity of piperifen. Experimental results show that the piperifoside can effectively inhibit infection of the SARS-CoV-2 on the susceptible cells, is relatively low in cytotoxicity, is expected to be used as a medicine for effectively resisting SARS-CoV-2 infection, and has an application prospect.
Owner:THE NAVAL MEDICAL UNIV OF PLA
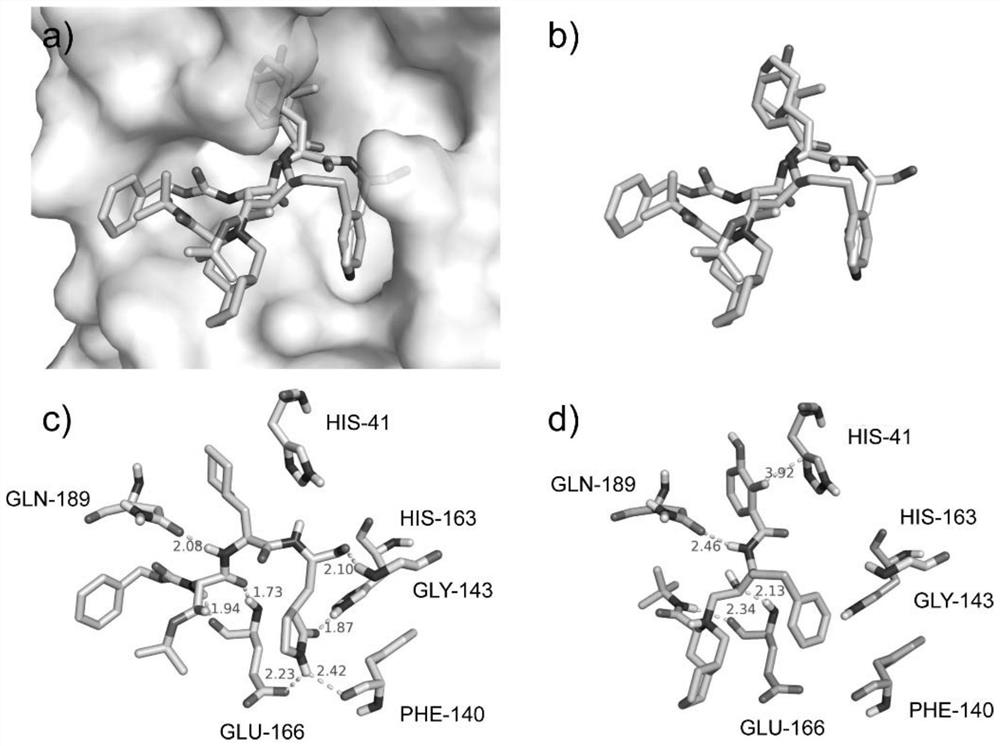
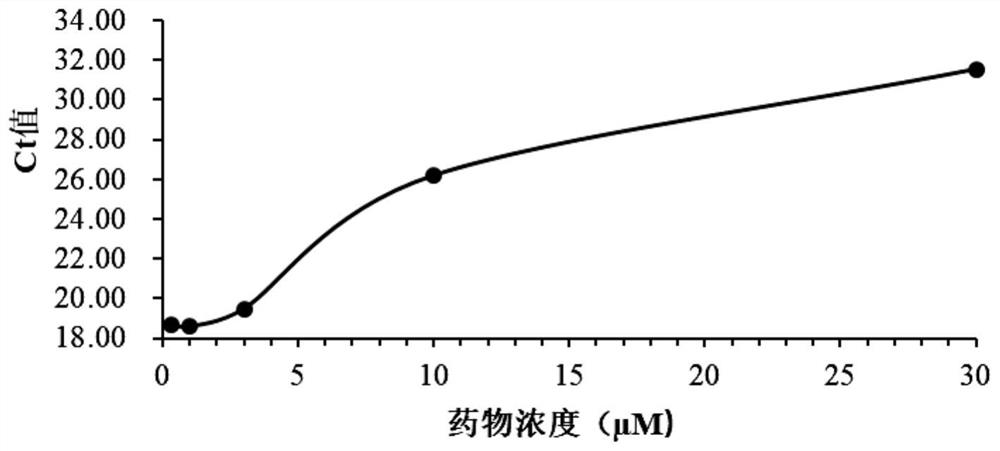
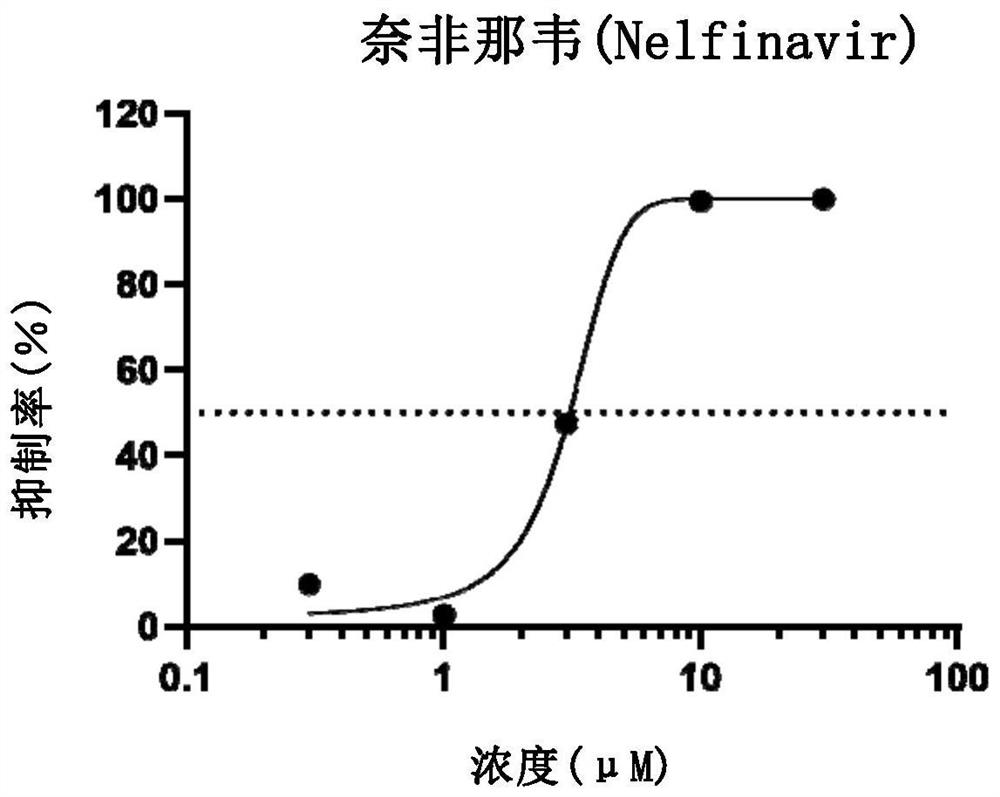
Application of nelfinavir in preparation of medicine for preventing and treating COVID-19
The invention relates to application of nelfinavir in preparation of a medicine for preventing and treating COVID-19. Specifically, the invention relates to application of nelfinavir and a pharmaceutical composition thereof as a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or 2019-nCoV) inhibitor in preparation of drugs for treating and / or preventing and relieving respiratory tract infection, pneumonia and other related diseases caused by 2019-nCoV infection.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +3
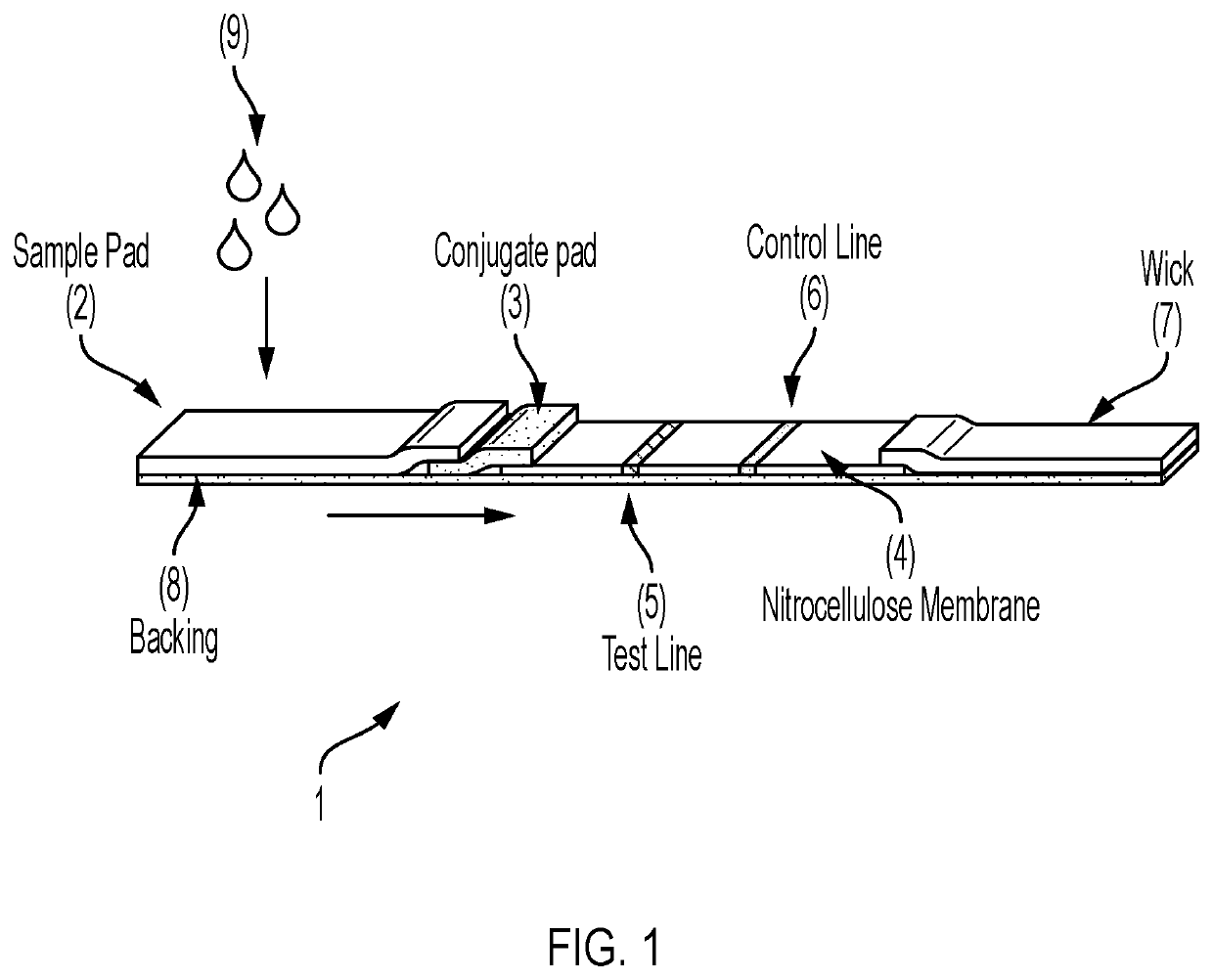
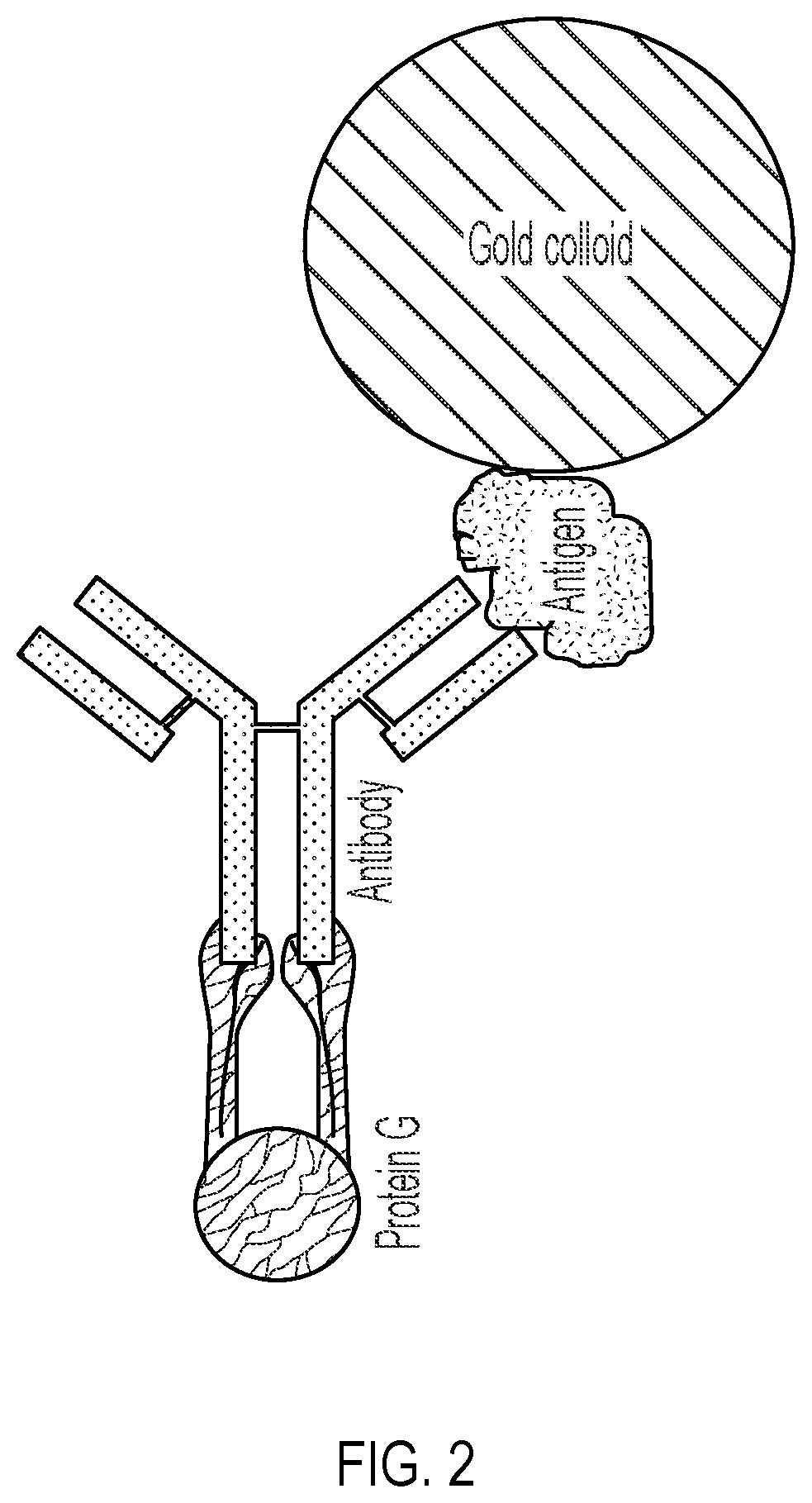
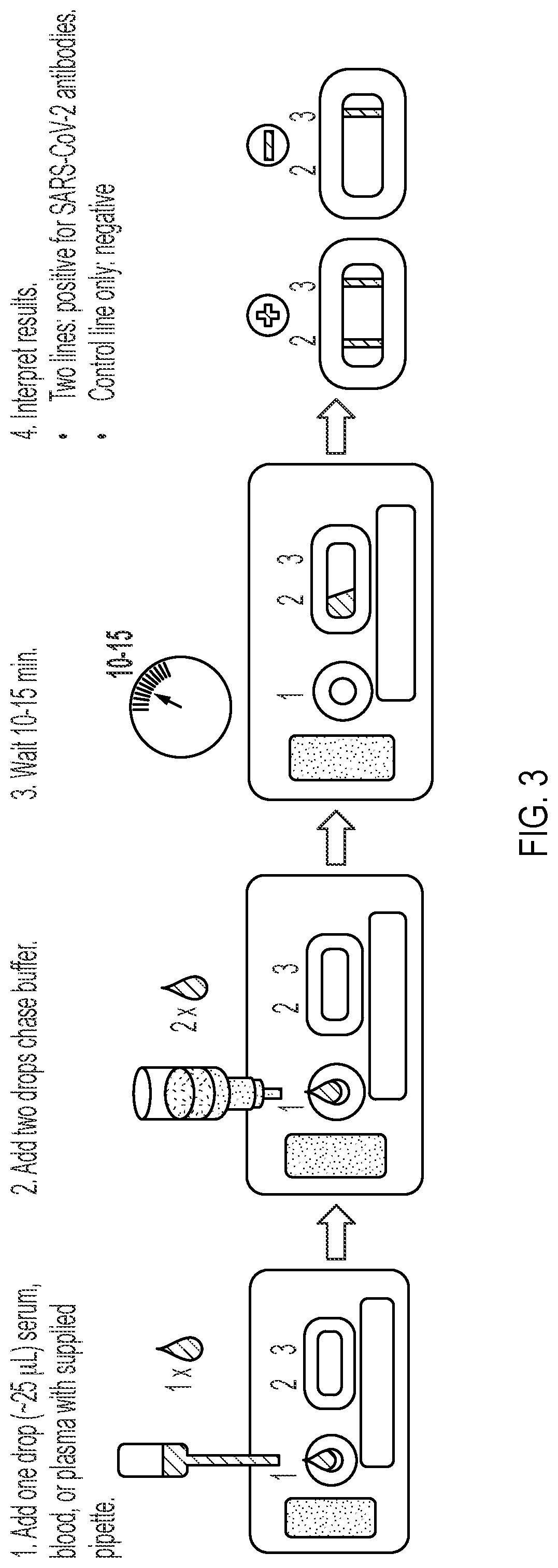
Lateral Flow Device for Detecting SARS-CoV-2 Antibodies in Human and Animal Samples
The invention provides a lateral flow device and methods for the detection of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a sample of bodily fluid of an animal or human. The test methods include contacting the sample with a conjugate comprising a recombinant SARS-CoV-2 spike protein antigen that has been conjugated to a detection agent, wherein an antigen-antibody complex is formed between the SARS-CoV-2 spike protein antigen conjugate and SARS-CoV-2 antibodies present in the sample; capturing the formed antigen-antibody complex with an Fc-binding molecule; and detecting the captured complex.
Owner:ZOETIS SERVICE LLC
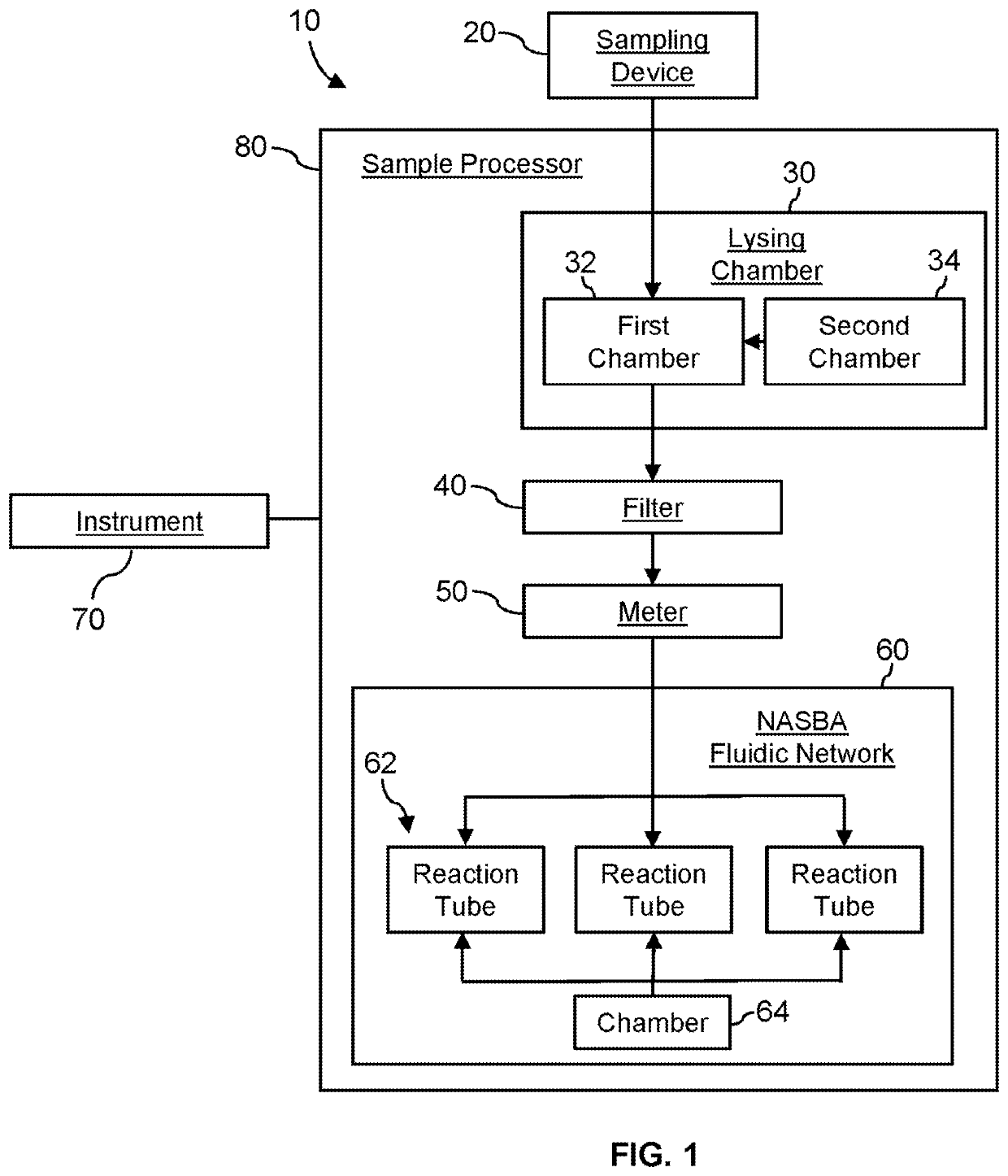
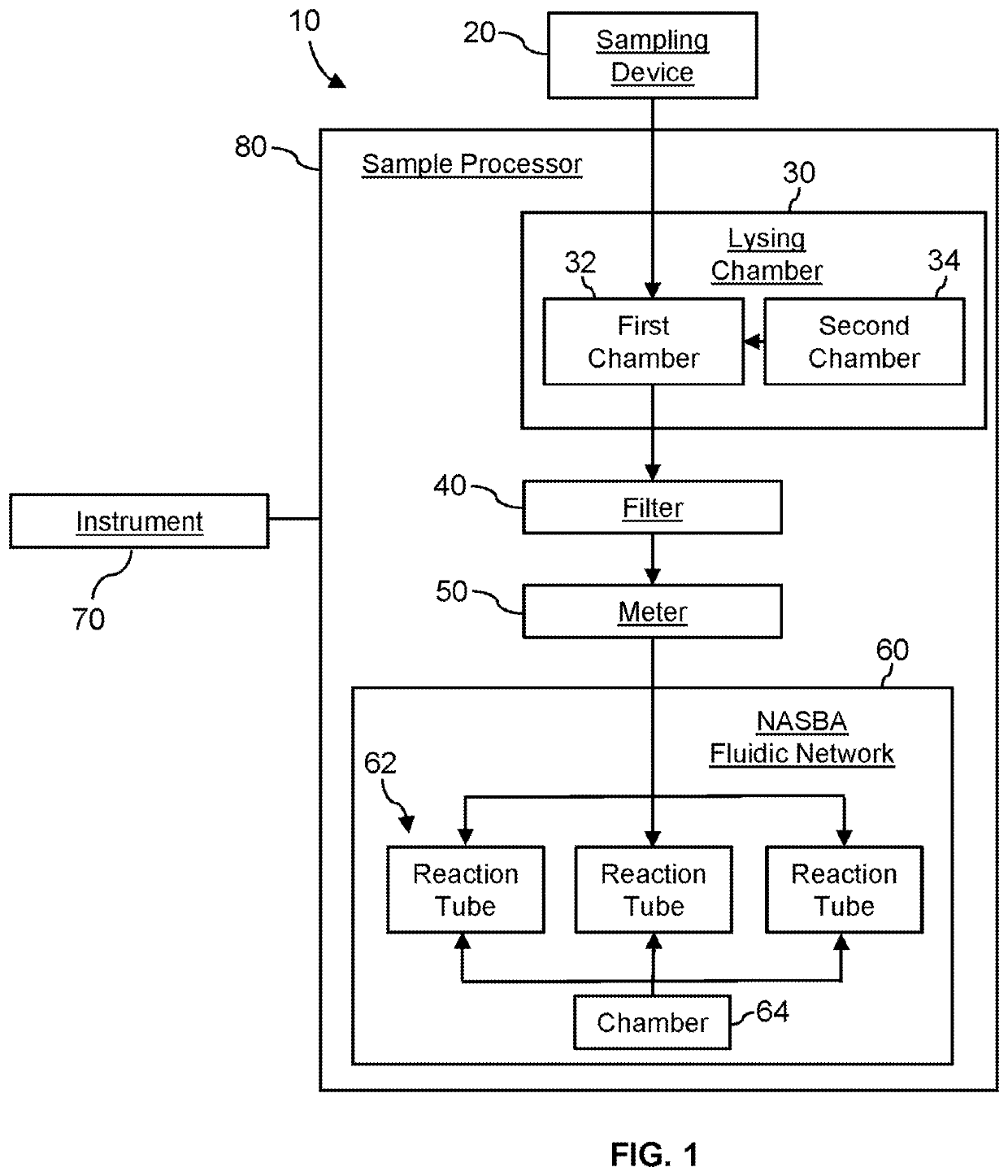
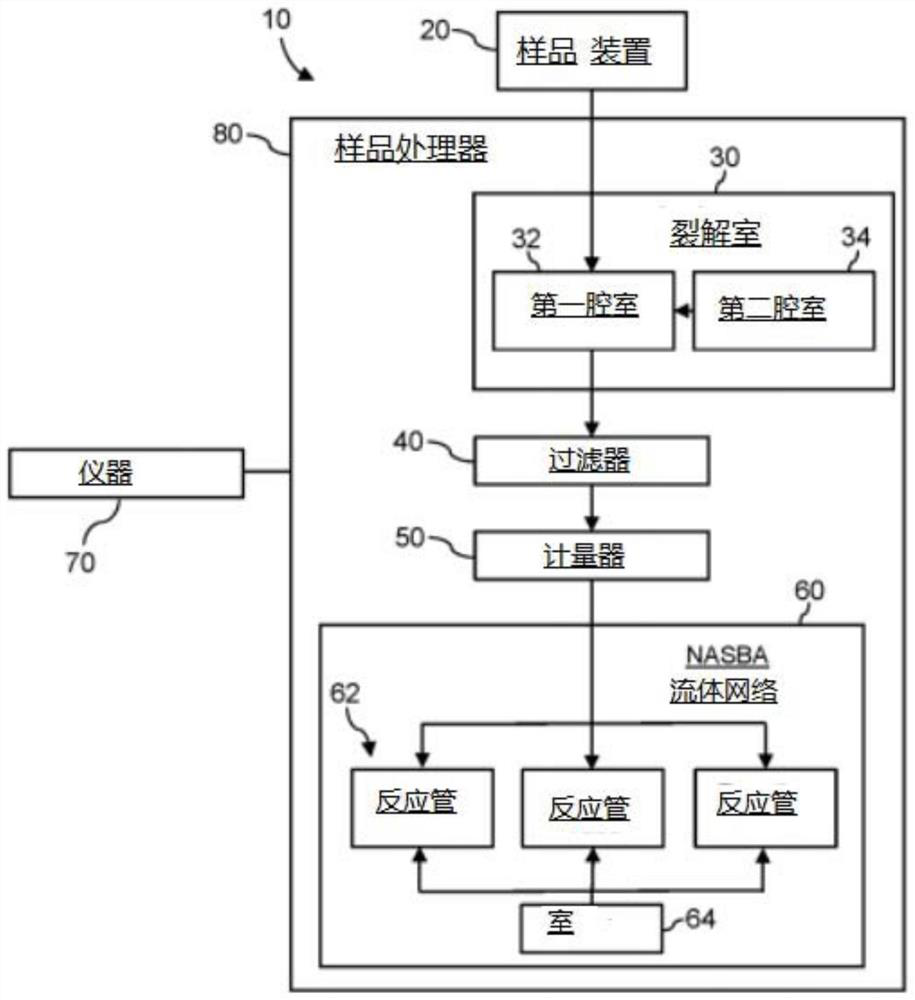
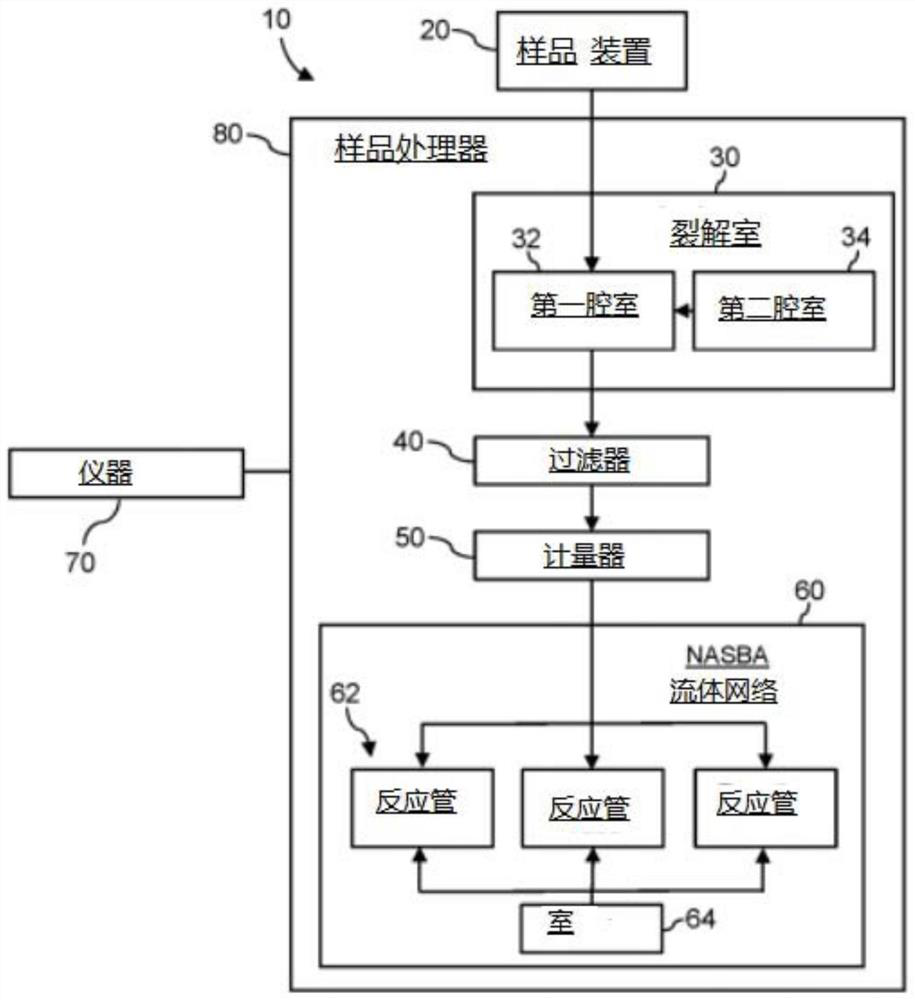
System for identifying severe acute respiratory syndrome corona virus 2 (sars-cov-2) ribonucleic acid (RNA)
InactiveUS20210129144A1Microbiological testing/measurementLaboratory glasswaresForward primerFluorophore
A system for detecting the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a biological sample includes a sampling device, a lysing chamber, a NASBA fluidic network, and an analytical instrument. The sampling device is configured to contain a sample containing a pathogen target sequence for SARS-CoV-2. The lysing chamber is configured to be in fluid communication with the sampling device to receive the sample. The is lysing chamber is configured to lyse the sample into a lysate. The NASBA fluidic network is configured to be in fluid communication with the lysing chamber to receive the lysate. The NASBA fluidic network includes an enzyme, a forward primer, and a reverse primer for amplifying a predetermined genetic sequence in the pathogen target sequence contained within the lysate. The forward primer has the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13, and SEQ ID NO: 17. The reverse primer has the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14, and SEQ ID NO: 18. A molecular beacon is configured to attach to the pathogen target sequence. The beacon has the oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19 and a fluorophore. The analytical instrument is configured to excite the beacon when the molecular beacon is attached to the pathogen target sequence to signal a presence of the pathogen target sequence.
Owner:TELEFLEX MEDICAL INC
Anti-spike glycoprotein antibodies and the therapeutic use thereof
ActiveUS20220251174A1Immunoglobulins against virusesAntiviralsGlycoproteinSevere acute respiratory syndrome coronavirus RNA
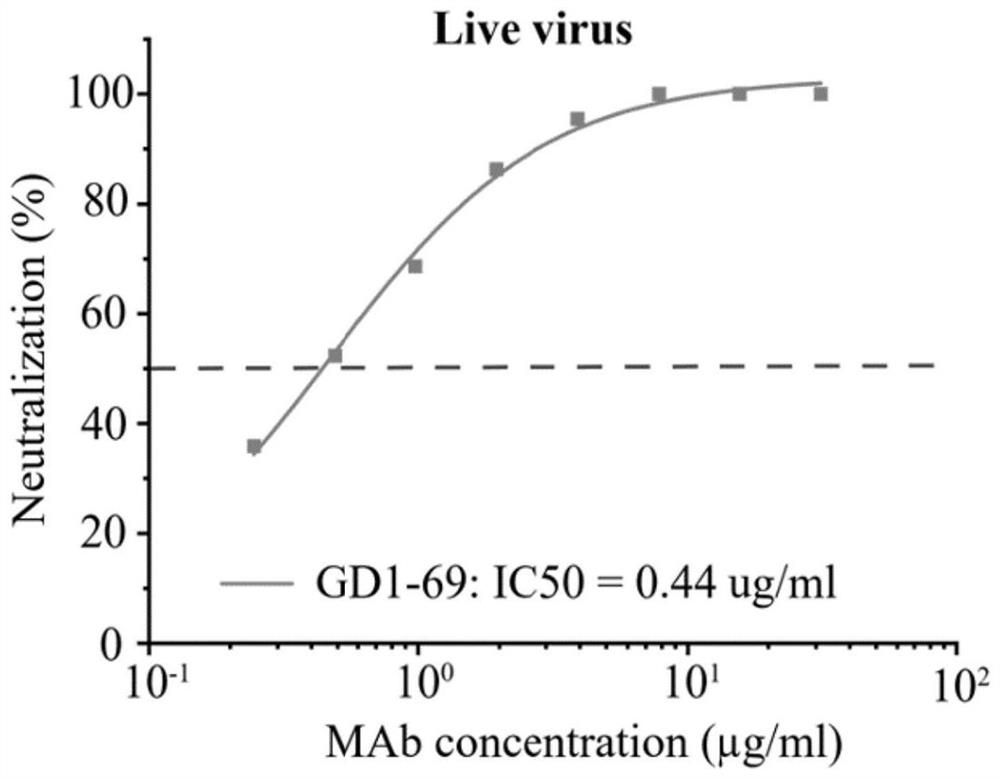
The present application relates to neutralizing antibodies or antigen-binding fragments thereof against betacoronaviruses such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), to nucleic acid(s) encoding such neutralizing antibodies or antigen-binding fragments thereof, and to mixture and compositions comprising such antibodies, antigen-binding fragments or nucleic acids. Such neutralizing antibodies or antigen-binding fragments thereof are able to block betacoronavirus entry into cells and / or to induce complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and / or antibody-dependent cellular phagocytosis (ADCP) against betacoronavirus-infected cells. Methods and uses of the antibodies, antigen-binding fragments thereof, nucleic acid(s) or compositions, including therapeutic, diagnostic, and preventative methods and uses for betacoronavirus infections and related diseases such as COVID-19, are also described.
Owner:TALEM THERAPEUTICS LLC
Antibody, preparation method thereof, coding nucleic acid and engineering cell
PendingCN114349850AStrong specificityIncreased sensitivityImmunoglobulins against virusesFermentationSevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2)Virology
The present disclosure relates to a method for preparing an antibody, an antibody, a nucleic acid encoding the antibody, and an engineered cell. The antibody provided by the invention can resist severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and is good in specificity, high in sensitivity and excellent in pathogen neutralization effect.
Owner:蒋庆华 +3
Detection system for severe acute respiratory syndrome coronavirus 2
The invention relates to a detection system for severe acute respiratory syndrome coronavirus 2. The detection system comprises a sampling device, a cracking chamber, an NASBA fluid network and an analysis instrument. The sampling device is configured to contain a sample containing a pathogen target sequence for SARS-CoV-2. The NASBA fluid network includes an enzyme, a forward primer and a reverse primer for amplifying a predetermined gene sequence in a pathogen target sequence contained within the lysate. The forward primer has an oligonucleotide sequence selected from the following groups: SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13 and SEQ ID NO: 17. The reverse primer has oligonucleotide sequences selected from the following groups: SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14 and SEQ ID NO: 18. The molecular beacon is configured to attach to a pathogen target sequence. The beacon has an oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19 and a fluorophore.
Owner:TELEFLEX MEDICAL INC
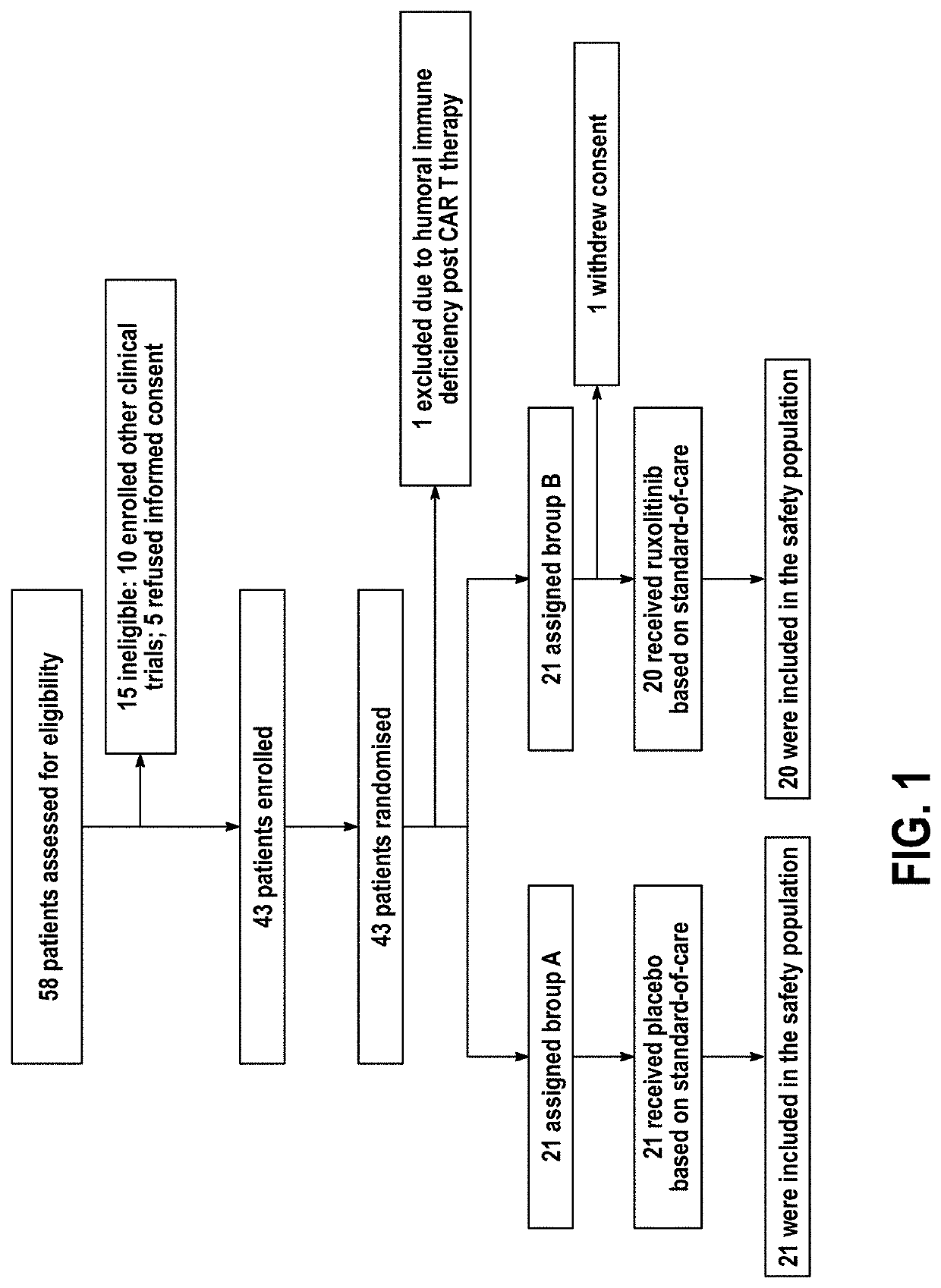
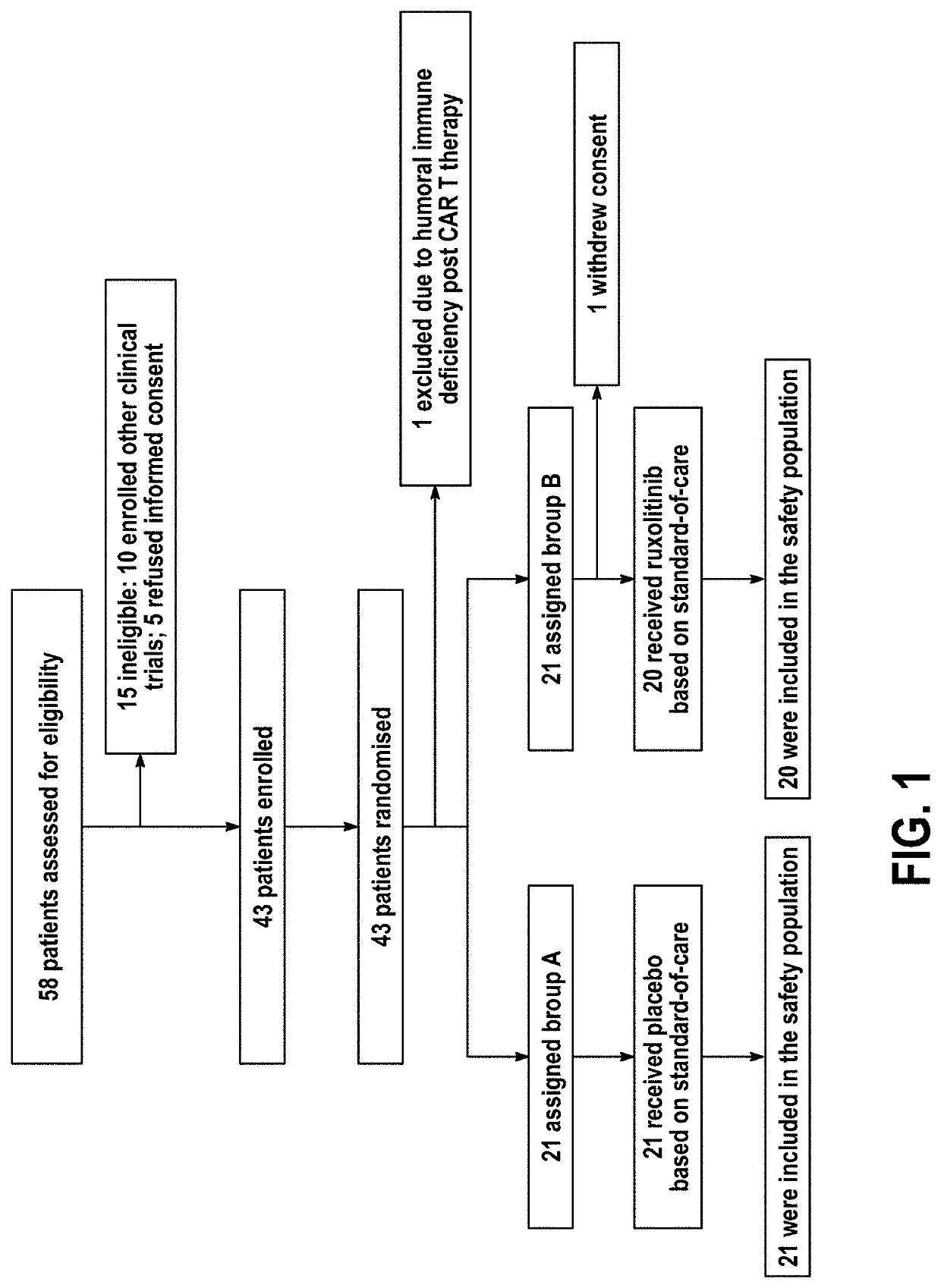
Compositions and methods for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection
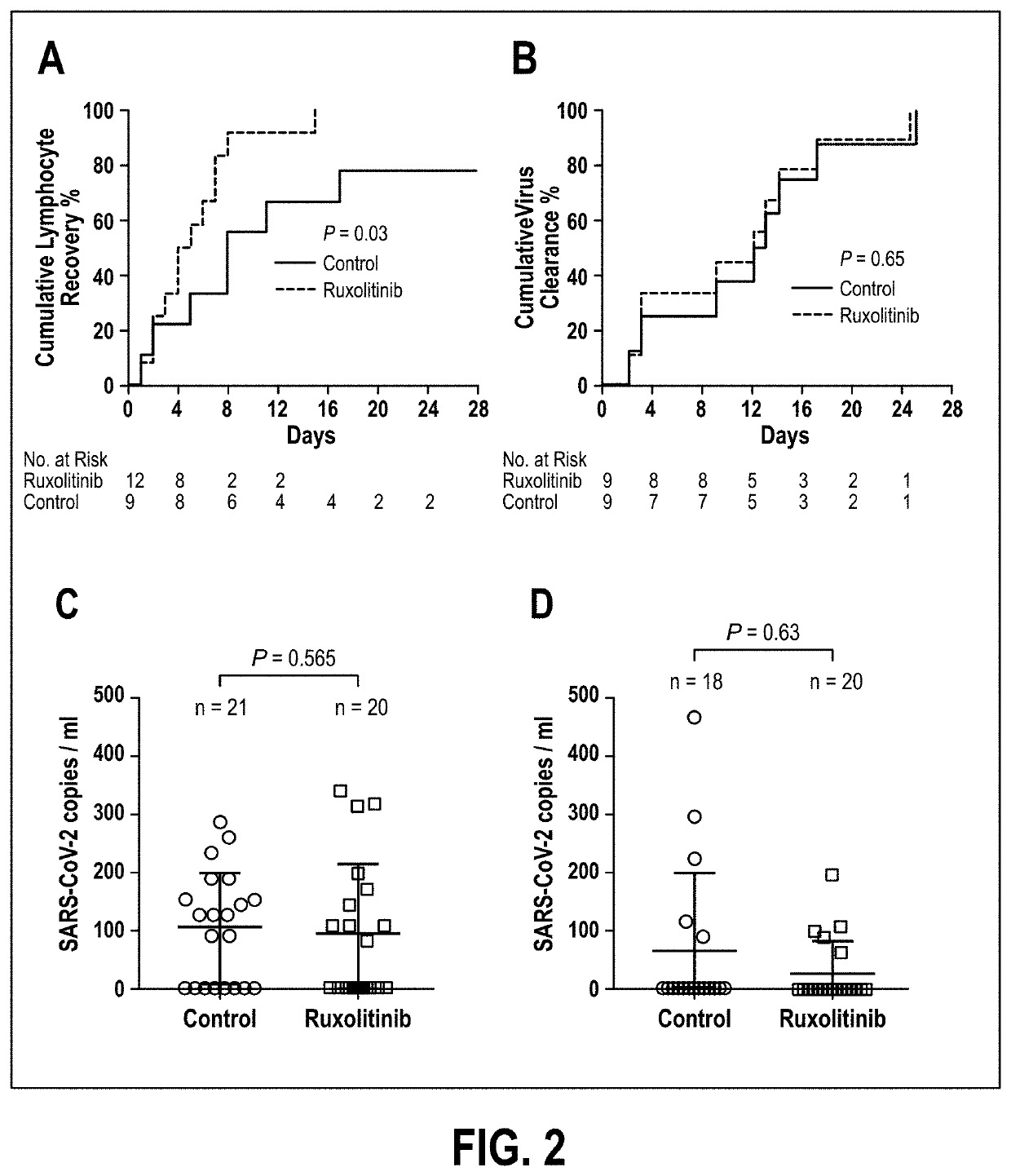
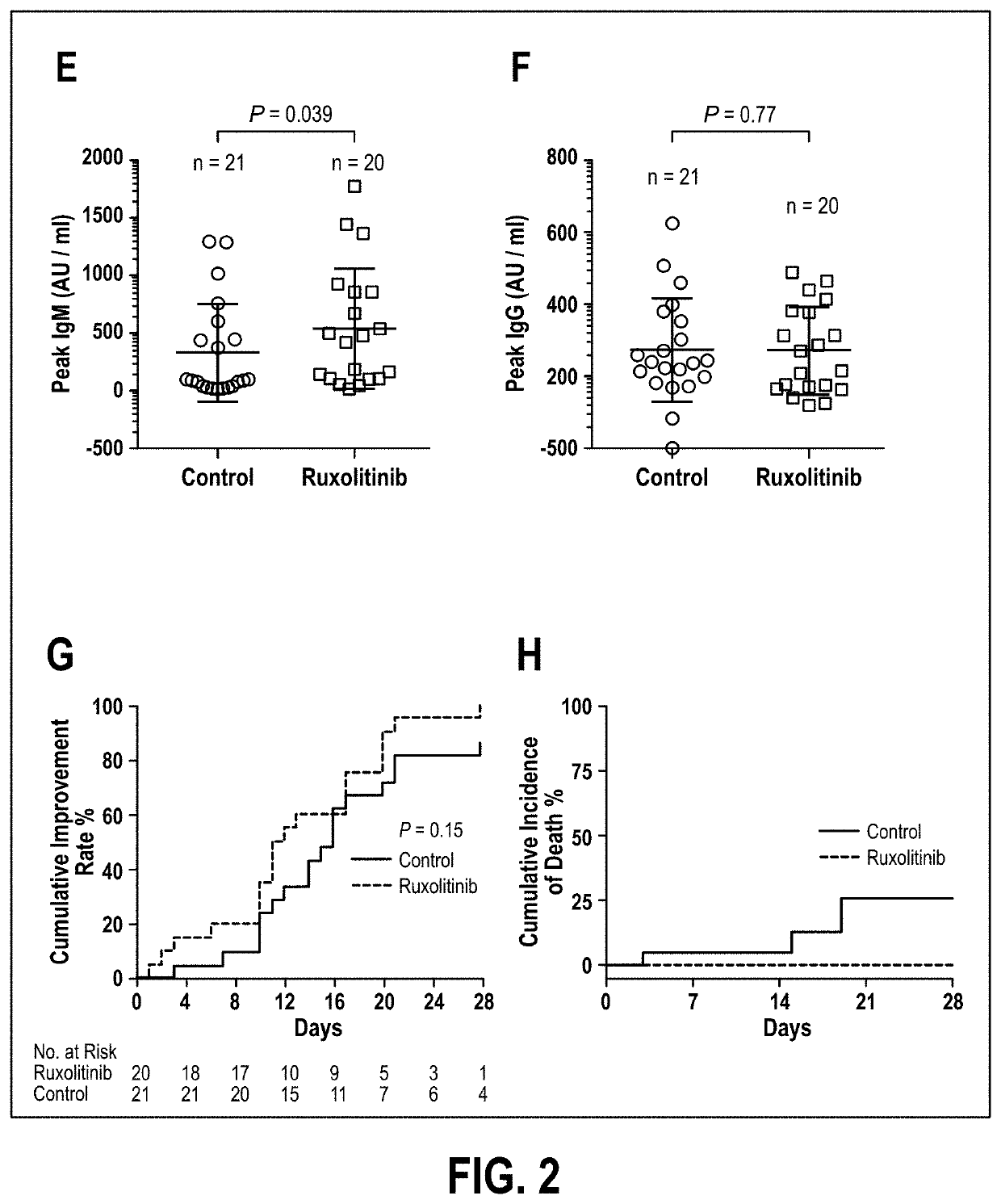
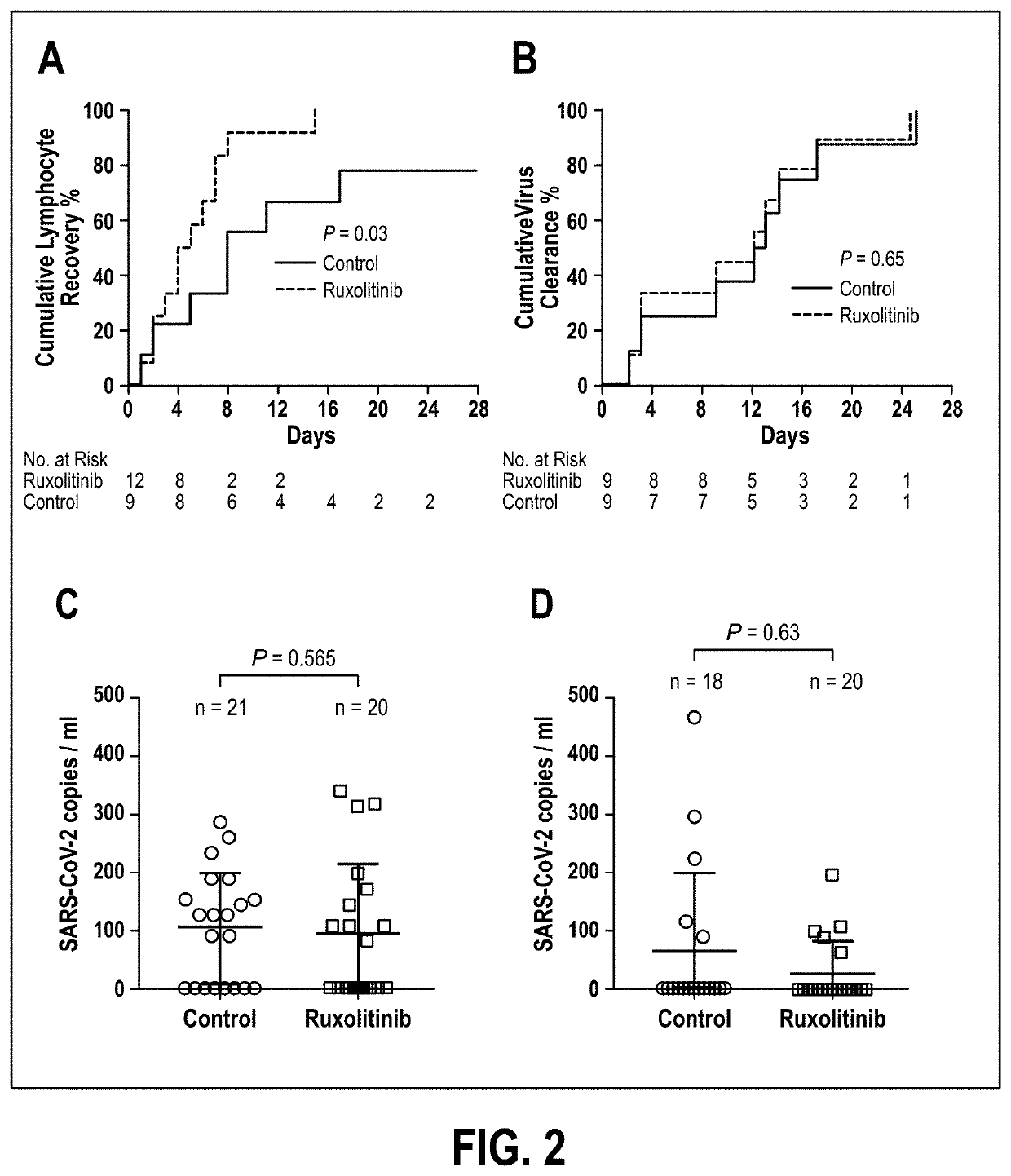
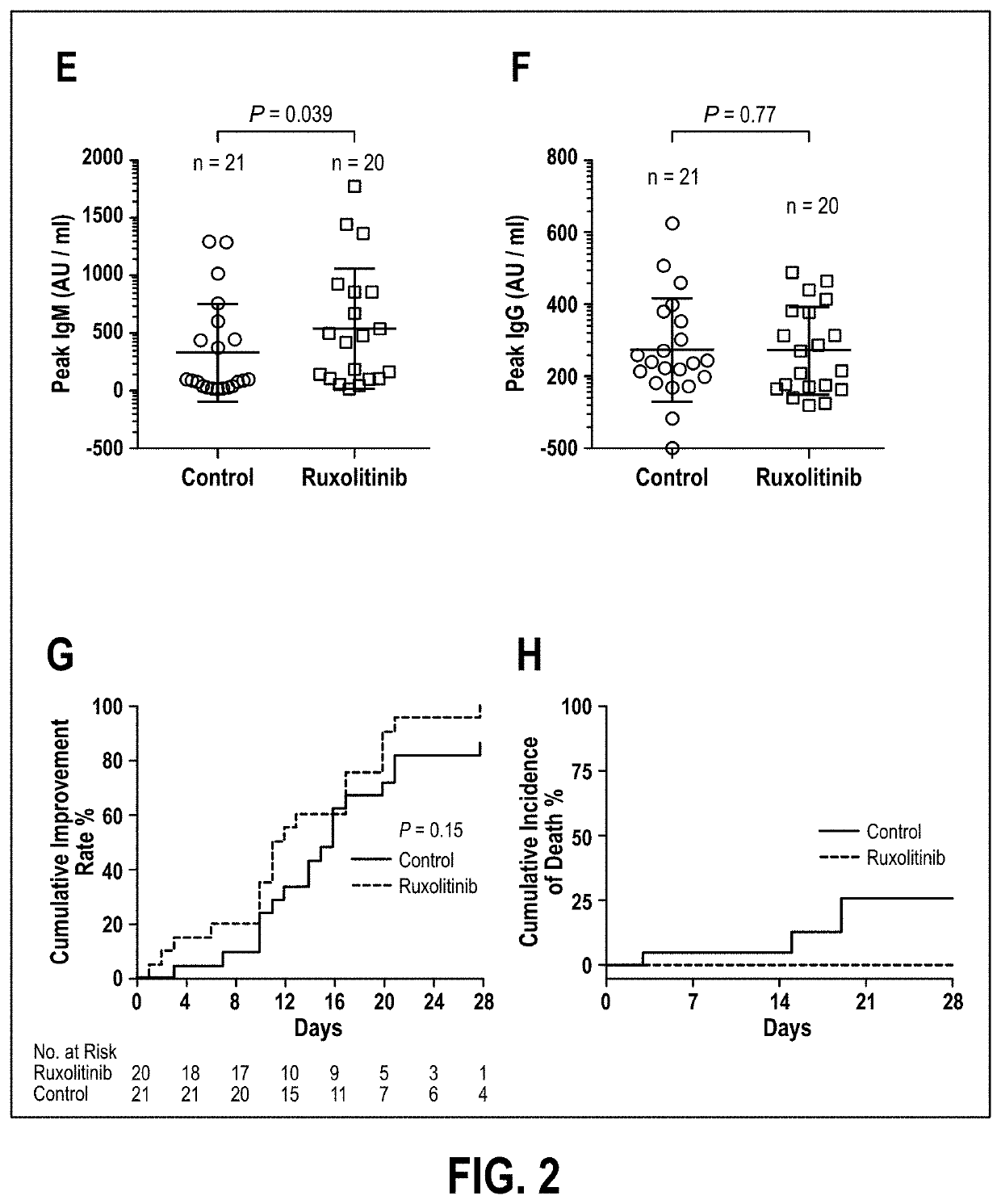
Disclosed herein are methods for treating an individual having a Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. The method may comprise administering a JAK inhibitor, for example ruxolitinib (JAKAFI®), to an individual in need thereof, such individual generally being an individual having, or suspecting of having, SARS-CoV-2 infection. The individual in need thereof may be an individual having, or suspected of having or at risk for developing SARS-CoV-2 infection-related cytokine storm. The individual in need thereof may further be an individual having, or suspected of having SARS-CoV-2 infection-related pneumonia.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Recombinant antibodies, kits comprising the same, and uses thereof
PendingUS20210341478A1Well formedBiological material analysisImmunoglobulins against virusesAntiendomysial antibodiesCapsid
Disclosed herein is a recombinant antibody against the nucleocapsid protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A kit containing the recombinant antibody for use in coronavirus detection is encompassed in the present disclosure. Also disclosed herein are in vitro methods of detecting coronavirus infection in a subject with the aid of the recombinant antibody.
Owner:ANTAIMMU BIOMED CO LTD
Method for detecting presence of severe acute respiratory syndrome coronavirus 2 in biological sample
The present invention relates to a method for detecting the presence of SARS-CoV-2 in a biological sample, comprising: subjecting the biological sample to cleavage to form a lysate; generating an amplified lysate by nucleic acid sequence (NASBA)-based amplification of a target nucleic acid sequence in the lysate in the presence of a forward primer having an oligonucleotide sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 5, SEQ ID NO: 9, SEQ ID NO: 13 and SEQ ID NO: 17, a reverse primer, and a molecular beacon; the reverse primer has an oligonucleotide sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 6, SEQ ID NO: 10, SEQ ID NO: 14 and SEQ ID NO: 18; the molecular beacon has an oligonucleotide sequence selected from the group consisting of SEQ ID NO: 3, SEQ ID NO: 7, SEQ ID NO: 11, SEQ ID NO: 15, and SEQ ID NO: 19, and a fluorophore; exposing the amplified lysate to an excitation source; detecting fluorescence of a fluorophore in the amplified lysate exposed to the excitation source; in response to detecting the fluorescence of the fluorophore, it is determined whether SARS-CoV-2 is present in the biological sample.
Owner:TELEFLEX MEDICAL INC
Application of small molecule compound pimavanserin to preparation of medicine for resisting SARS-CoV-2
ActiveCN113797200ALow toxicityAvoid infectionOrganic active ingredientsAntiviralsGreen monkeyBULK ACTIVE INGREDIENT
The invention discloses an application of a small molecule compound pimavanserin to preparation of a medicine for resisting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The medicine for resisting the SARS-CoV-2 is a medicine composition which takes the pimavanserin as a unique active ingredient or contains the pimavanserin, and the medicine for resisting the SARS-CoV-2 refers to a medicine for preventing or treating SARS-CoV-2 infection. According to the invention, a susceptible cell line of the SARS-CoV-2, including an African green monkey kidney cell Vero E6 and a human lung adenocarcinoma cell Calu-3, is utilized to detect the anti-SARS-CoV-2 activity of the pimavanserin. Experimental results show that the pimavanserin can effectively inhibit infection of the SARS-CoV-2 on the susceptible cells, is relatively low in cytotoxicity, is expected to be used as a medicine for effectively resisting SARS-CoV-2 infection, and has an application prospect.
Owner:THE NAVAL MEDICAL UNIV OF PLA
Compositions and methods for the treatment of severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection
Disclosed herein are methods for treating an individual having a Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. The method may comprise administering a JAK inhibitor, for example ruxolitinib (JAKAFI®), to an individual in need thereof, such individual generally being an individual having, or suspecting of having, SARS-CoV-2 infection. The individual in need thereof may be an individual having, or suspected of having or at risk for developing SARS-CoV-2 infection-related cytokine storm. The individual in need thereof may further be an individual having, or suspected of having SARS-CoV-2 infection-related pneumonia.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com