Synthesis method of cefixime
A synthetic method, the technology of cefixime, applied in the field of drug synthesis, can solve the problems of difficult separation of by-product sulfide, low reaction yield, and large environmental impact, and achieve easy separation, shortened reaction steps, reduced energy consumption and pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
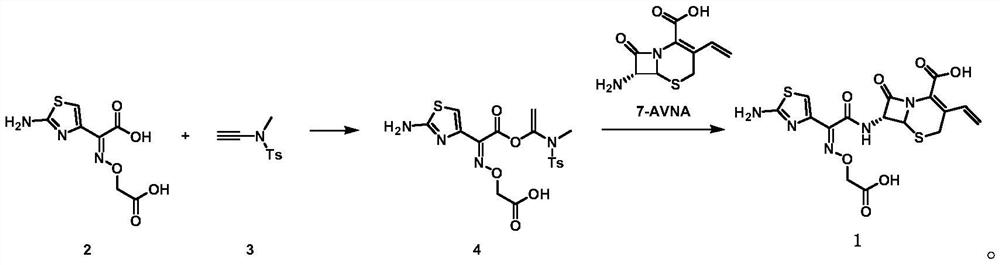
[0025] The preparation of embodiment 1 compound cefixime (1)
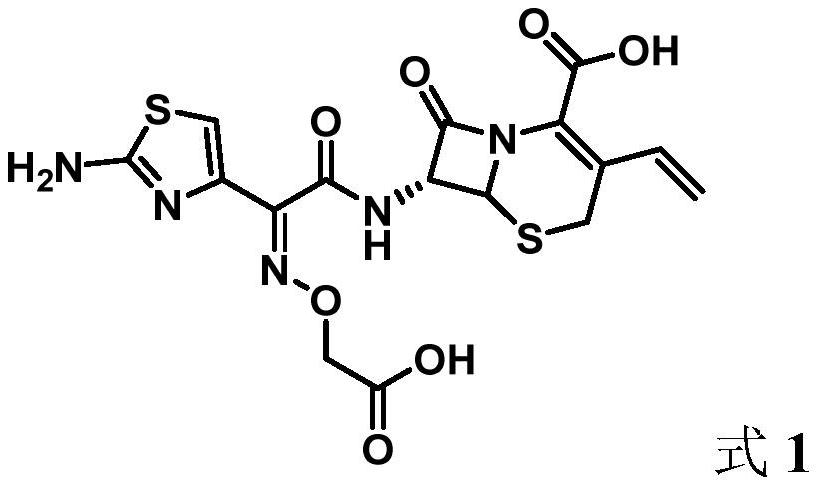
[0026] At room temperature (25°C), 4.90 g (20 mmol) of aminothiazole acetoxamic acid analog (2) and 4.20 g (20 mmol) of compound (3) were added to a 250 ml round-bottomed flask, and 100 ml of dichloromethane was added and stirred until Compound (3) was completely reacted. Compound 7-AVNA 5.00 g (22 mmol) was added to the above solution and stirring was continued at room temperature in air until the intermediate (4) was completely reacted. The reaction mixture was concentrated and purified by silica gel column chromatography to obtain 8.52 g of cefixime (1) in 94.0% yield with 99.4% HPLC purity.
[0027] 1 H NMR (600MHz, Methanol-D 4 )δ: 7.12(q, J=28.9Hz, 1H), 6.96(s, 1H), 5.56(d, J=18.0Hz, 1H), 5.88(d, J=4.7Hz, 1H), 5.32(d, J=11.4Hz, 1H), 5.21 (d, J=4.8Hz, 1H), 4.75 (s, 2H), 3.78 (d, J=17.5Hz, 1H), 3.61 (d, J=17.4Hz, 1H) . 13 C NMR (600MHz, Methanol-D 4 )δ: 174.0, 171.5, 165.3, 164.2, 150.2, 141.2, 133.4, 12...
Embodiment 2
[0028] The preparation of embodiment 2 compound cefixime (1)
[0029] At room temperature (25°C), 4.90 g (20 mmol) of aminothiazole acetoxamic acid analog (2) and 4.20 g (20 mmol) of compound (3) were added to a 250 ml round-bottomed flask, and 100 ml of tetrahydrofuran was added and stirred until the compound ( 3) Completely reacted. Compound 7-AVNA 5.00 g (22 mmol) was added to the above solution and stirring was continued at room temperature in air until the intermediate (4) was completely reacted. The reaction mixture was concentrated and purified by silica gel column chromatography to obtain 7.56 g of cefixime (1) in 83.4% yield with 99.0% HPLC purity.
Embodiment 3
[0030] The preparation of embodiment 3 compound cefixime (1)
[0031] At room temperature (25°C), 4.90 g (20 mmol) of aminothiazole acetoxamic acid analog (2) and 4.20 g (20 mmol) of compound (3) were added to a 250 ml round-bottomed flask, and 100 ml of toluene was added and stirred until the compound ( 3) Completely reacted. Compound 7-AVNA 5.00 g (22 mmol) was added to the above solution and stirring was continued at room temperature in air until the intermediate (4) was completely reacted. The reaction mixture was concentrated and purified by silica gel column chromatography to give 7.87 g of cefixime (1) in 86.8% yield with 98.9% HPLC purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com