Recombinant engineering bacterium capable of efficiently expressing plectasin and application of recombinant engineering bacterium
A technology of recombining engineering bacteria and mycelia, which is applied in the field of genetic engineering, can solve the problems of unsatisfactory expression, high cost, and high impurity content, and achieve good application prospects, increased expression, and significant effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Design of a T2A peptide-based tetraploid pleomycin tetramer
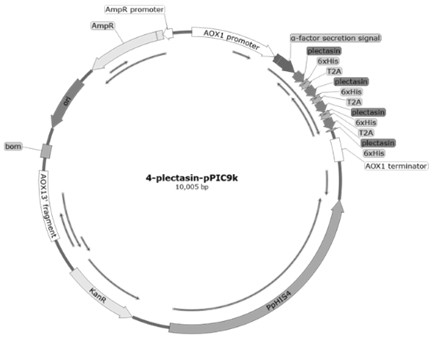
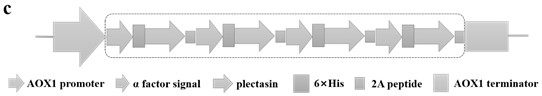
[0041] Based on the obtained gene sequence of pectin and the gene sequence of T2A peptide (GenBank: UER86417.1). A 6×His tag was added to the N-terminus of each pectin for purification. The T2A peptide sequence was added to the C-terminus of plucuronidin to connect with the next set of gene expression cassettes, and in this way, a quadruple concatemer of plucuronidin was constructed (the construction diagram is shown in Fig. figure 1 , 2 shown). The base sequence was optimized according to the codon usage preference of Pichia pastoris, and the whole gene was synthesized by Beijing Huada Gene Technology Co., Ltd. The nucleotide sequence of the optimized quadruple concatemer is shown in SEQ ID NO.5, wherein, the gene of pectin is shown in SEQ ID NO.1, encoding 40 amino acids, as shown in SEQ ID NO.2 .
[0042] SEQ ID NO. 5:
[0043]5’-ATGAGATTTCCTTCAATTTTTACTGCAGTTTTATTCGCAGCATCCTCCGCATTAGCTGCTC...
Embodiment 2
[0044] Example 2 Cloning of plectomycin gene
[0045] Using the above-synthesized tetraploid gene fragment of phleomycin as a template, primers for seamless ligation were designed upstream and downstream of the phlegomycin gene, and PCR amplification of the phlegomycin gene fragment was performed.
[0046] The sequences of primers are as follows:
[0047] Upstream primer: 5'-GCTACGTAGAATTCGGTTTCGGTTGTAAC-3', as shown in SEQ ID NO.6;
[0048] Downstream primer: 5'-CGCTTAATGATGATGATGATGATGGTAGCACTTACAGACG-3', as shown in SEQ ID NO.7.
[0049] The primers for seamless ligation were designed upstream and downstream, and PCR was performed to amplify the gene fragment of the pPIC9k cloning vector.
[0050] The sequences of primers are as follows:
[0051] Upstream primer: 5'-CATCATCATTAAGCGGCCGCGAATTA-3', as shown in SEQ ID NO.8;
[0052] Downstream primer: 5'-GAATTCTACGTAAGCTTCAGCCTCTC-3' as shown in SEQ ID NO.9.
[0053] The PCR reaction system was: 2×PCR Buffer 25 μl, dNTP 1...
Embodiment 3
[0056] Example 3 Construction of the expression vector of the pecomycin gene
[0057] The pleomycin quadruple concatemer gene fragment was connected with the pPIC9k cloning vector using seamless cloning technology, and the ligated product was transferred into E.coli DH5α competent cells were plated on solid plates of (LB) medium containing 50 μg / mL kanamycin. After culturing in a 37°C incubator for 15 hours, pick a single clone into LB liquid medium containing 50 μg / mL kanamycin, and cultivate overnight in a 37°C shaker at a speed of 220 rpm. After positive verification, sequencing was performed, and the resulting expression vector was named as pPIC9k-Plectasin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com