Preparation method and application of NK (Natural Killer) cell for over-expressing CD16a
A NK cell and overexpression technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, cells modified by introducing foreign genetic material, etc., can solve the problems of low transfection efficiency, excessive cell death, etc. Survival rate, the effect of enhancing the killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
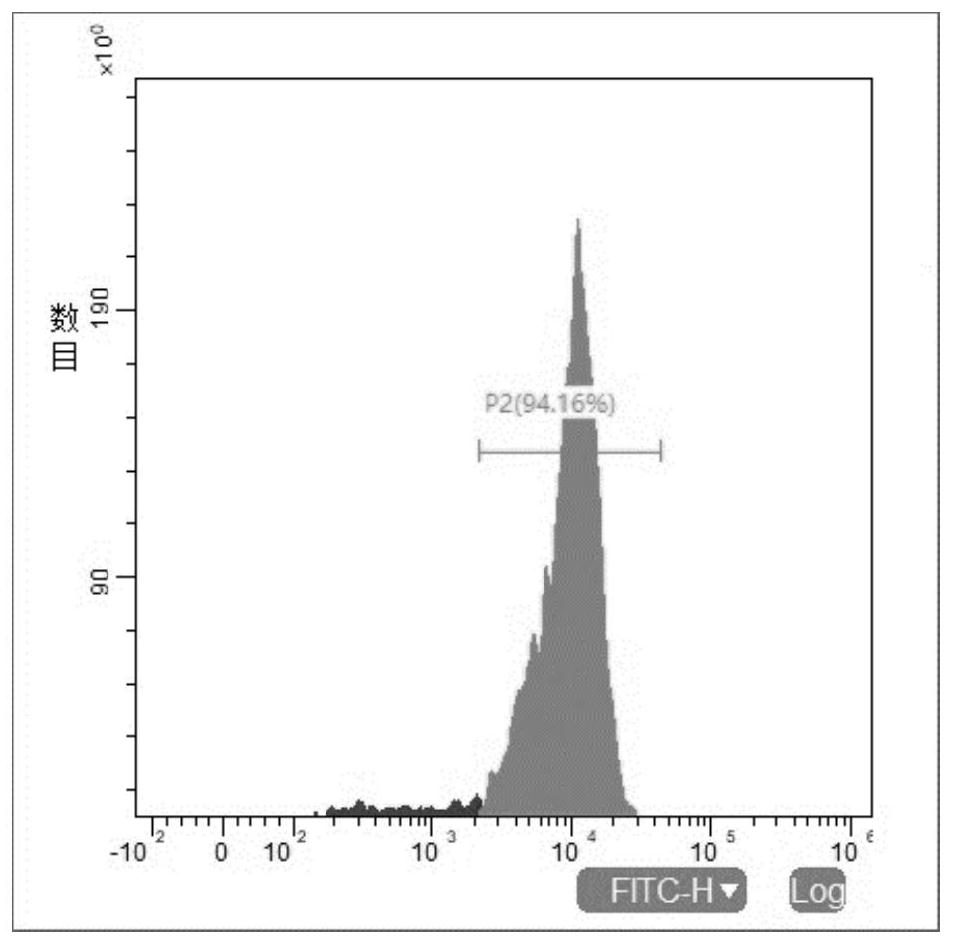
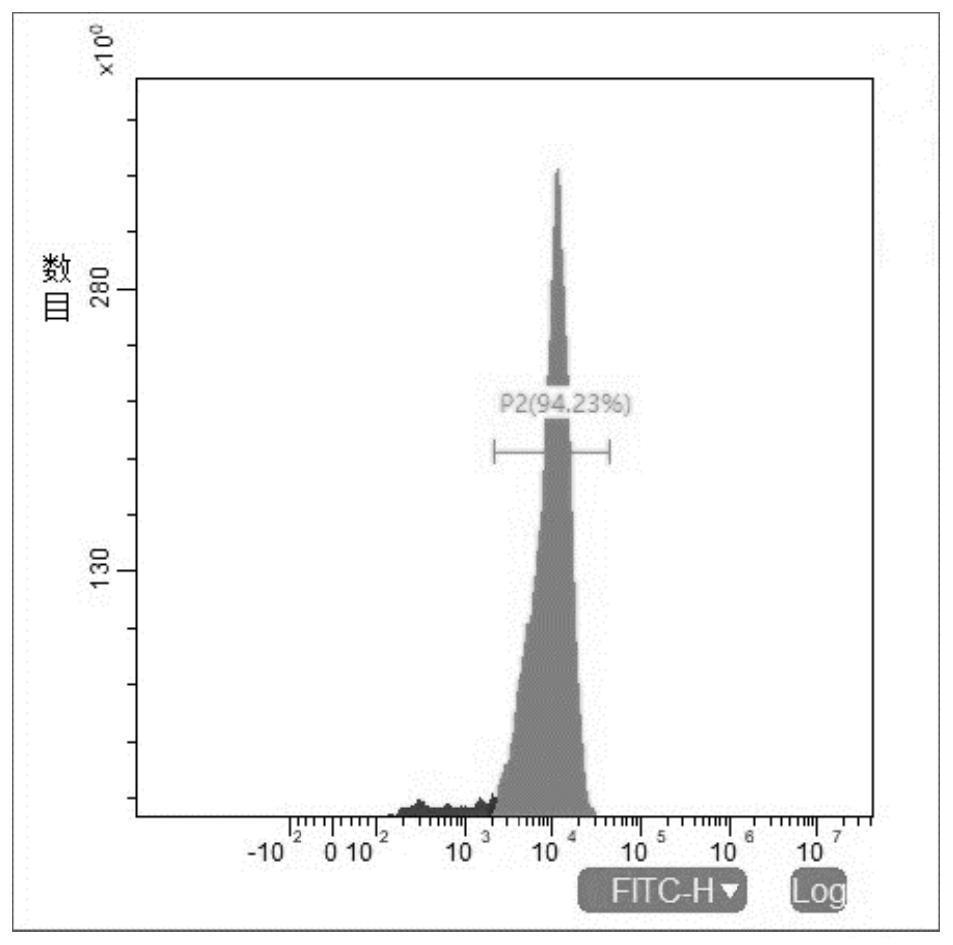
[0058] Example 1: Preparation of NK916 cells (NK92MI cells overexpressing CD16)
[0059] (1) Prepare a three-plasmid system. The three-plasmid system is a target plasmid for overexpressing CD16, a psPAX2 plasmid and a pMD2.G plasmid.
[0060] (2) Preparation of basic medium: DMEM complete medium containing 5-20 vol% fetal bovine serum and 1 vol% penicillin-streptomycin.
[0061] (3) about (1~10)×10 6 293T cells were inoculated into T75 culture flasks, basic medium was added, and cultured overnight. When the cell density reached 50-90%, the supernatant in the culture flask was aspirated, and 10 mL of DMEM containing only 0.1-2vol% fetal bovine serum was added. The culture medium starves the cells for 1-3 hours to obtain tool cells;
[0062] (4) Pipette the Opti-MEM medium in an amount of 1-10 mL per virus, and divide it into two equal parts. One part is added with lentiviral packaging plasmids psPAX2, pMD2.G and the target expression plasmid, and mixed evenly to obtain a pla...
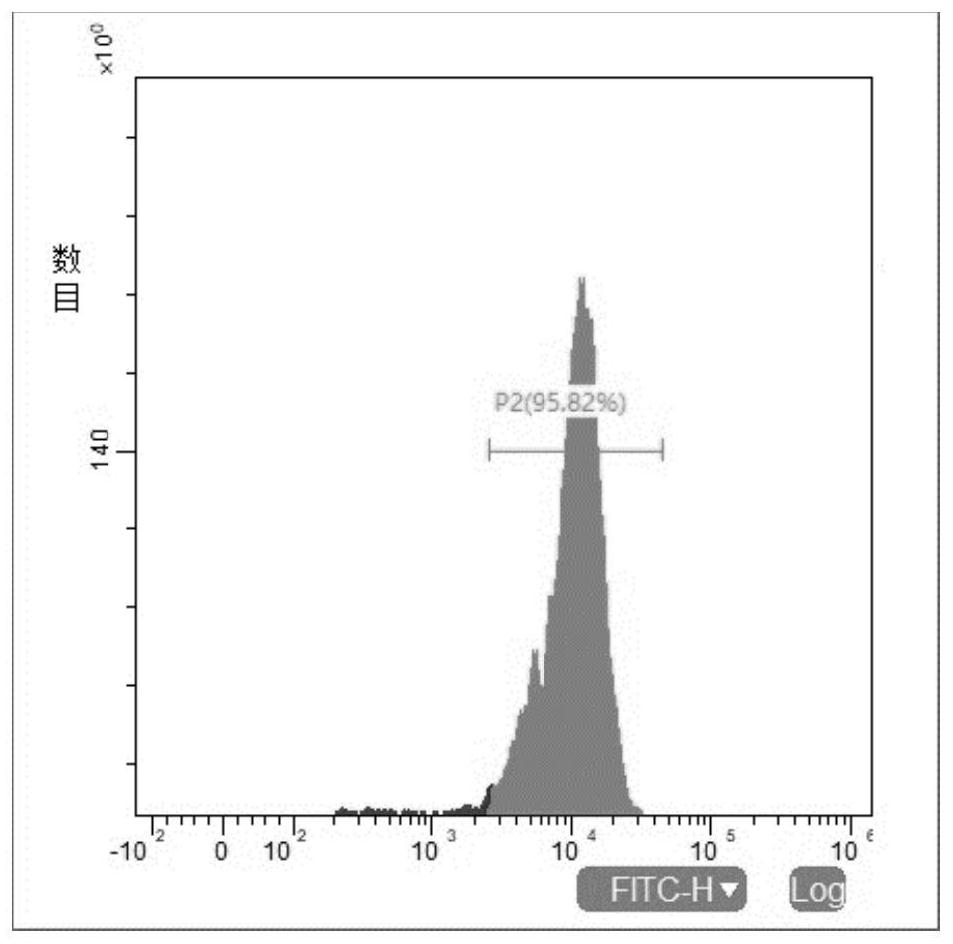
Embodiment 2
[0077] The operation mode of embodiment 2 is basically the same as that of embodiment 1, and the main difference is that the first, second and third culture medium in embodiment 1 are replaced as follows; and the mass ratio of pMD2.G, psPAX2, and the target expression plasmid The ratio of polyethyleneimine to the total amount of the three plasmids was 1:1, and the final concentration of polybrene was 8 μg / mL.
[0078] The specific composition of the first cell culture medium is as follows: 0.1 mM inositol, 0.2 mM folic acid, 0.1 mM mercaptoethanol, 2 vol % fetal bovine serum, 2 vol % horse serum, and 2 vol % double antibody are added to the MEM basal medium.
[0079] The specific composition of the second cell culture medium is as follows: 1 mM inositol, 0.05 mM folic acid, 0.1 mM mercaptoethanol, 25 vol% fetal bovine serum, 25 ol% horse serum, and 1 vol% double antibody are added to the MEM basal medium.
[0080] The specific composition of the third cell culture medium is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com