Compositions and methods for enhancing opioid receptor binding by opioid hexadienoic acid esters and optionally substituted hexadienoic acid esters
A technology of hexadienoate and methyl hexanoate, applied in the field of opioid-derived compositions, can solve the problem that naloxone cannot flow through the BBB quickly, and achieve the effect of lasting neutralization/awake effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
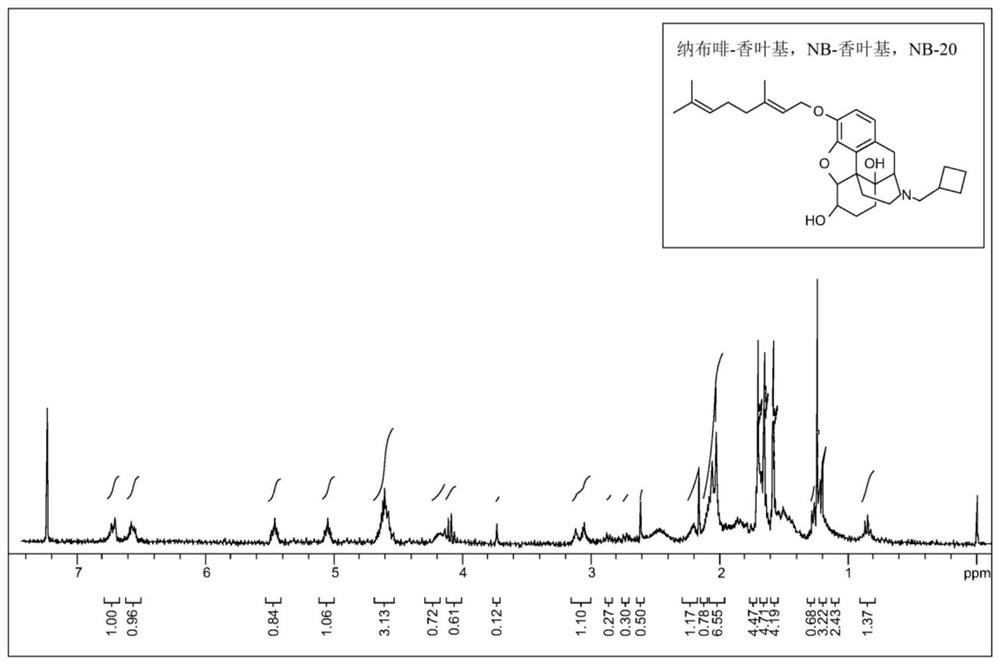
[0082] (E)-3-(cyclobutanemethyl)-9-((3,7-dimethyloctane-2,6-diethylenetriamine-1-yl)oxy)-1,2, 3,4,5,6,7,7a-Octahydro-4aH-4,12-methylbenzofuro[3,2-e]isoquinoline-4a,7-diol, nalbuphine-aroma Leaf base, (NB-20). To a suspension of nalbuphine hydrochloride (400 mg, 1.0 mmol) in acetone (20 mL) and toluene (20 mL) was added potassium bicarbonate (280 mg, 2.0 mmol) at room temperature. Geranyl bromide (320 mg, 1.5 mmol) was added. The reaction mixture was stirred under reflux conditions for 4 hours and at room temperature overnight. The reaction mixture was evaporated and the residue was purified by column chromatography (silica gel, EtOAc / heptane / methanol, 1:1:0.10). After evaporation of selected fractions, a colorless oil formed in 45% yield and 91% purity by HPLC. by NMR 1 H to confirm the structure.
[0083] 3-(Cyclobutanemethyl)-9-(((2E,6E)-3,7,11-trimethyldodecyl-2,6,10-triethylenetetramin-1-yl) oxy)-1,2,3,4,5,6,7,7a-octahydro-4aH-4,12-methylbenzofuro[3,2-e]isoquinoline...
example 2
[0103] Example 2 - Stability in simulated gastrointestinal fluid (sGIF).
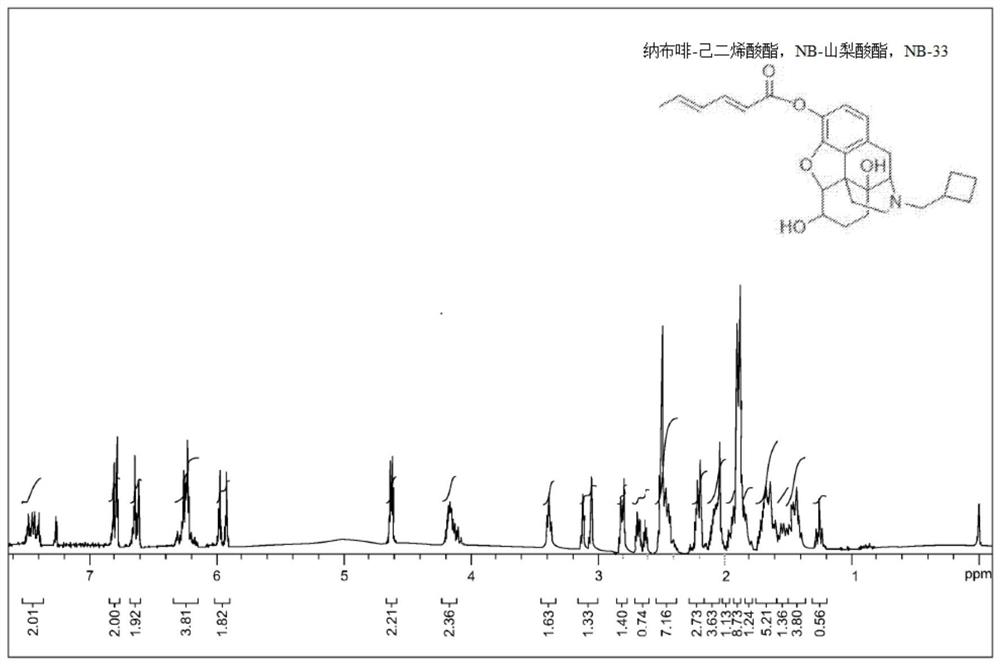
[0104] The stability evaluation of NB-33 in simulated gastrointestinal fluid (sGIF) is as follows, and Table 1 summarizes the data of each compound.
[0105] sGIF is 0.5% pepsin (Alfa Aesar, pepsin, porcine stomach) in 0.1 N HCl in water. Each derivative (50 mg) was mixed with sGIF (50 mL) and incubated on a shaker at a temperature of 37°C. The hydrolysis and release of nalbuphine was monitored by HPLC at T=0 hours, 0.5 hours, 1 hour, 2 hours and 4 hours. The acceptance criterion is that no less than 80% of the derivatives are still intact after 4 hours.
example 3-
[0106] Example 3- Stability in human plasma.
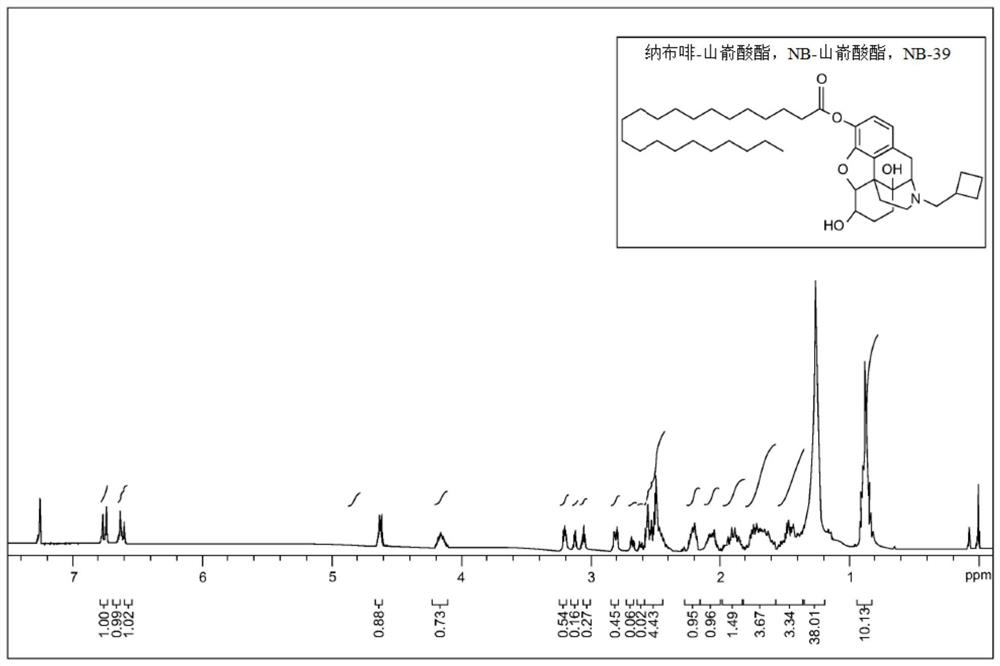
[0107] The stability of NB-56 in human plasma was assessed as follows, and Table 1 summarizes the data for each compound.
[0108] NB-56 (1.0 mg) was dissolved in 10 mL of plasma (pooled normal human plasma, sodium citrate, Innovative Research Corporation) while stirring at a temperature of 20°C for 10 minutes. The solution was incubated at 37°C. Take 1 mL of solution for each sample. MeCN (0.05 mL) was added to the sample solution. Shake for 1 minute and then centrifuge (15 minutes, 14.000 r / m). The supernatant was filtered off and extracted with EtOAc (2 x 20 mL). Use MgSO 4 The combined extracts were dried and concentrated in vacuo. The residue was dissolved in methanol (20 μl). The solution was used for HPLC injection.
[0109] The hydrolysis and release of nalbuphine was monitored by HPLC at T=0 hours, 0.5 hours, 1 hour, 2 hours and 4 hours. The acceptance criterion is that no less than 20% of the derivatives are h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com