Polyurethane-aspartic polyurea resin, preparation method thereof and polymer
A technology of aspartic polyurea resin and polyurethane, which is applied to the preparation of cyanide reaction, chemical instruments and methods, and the preparation of organic compounds. The loss of the effect of promoting the completion of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
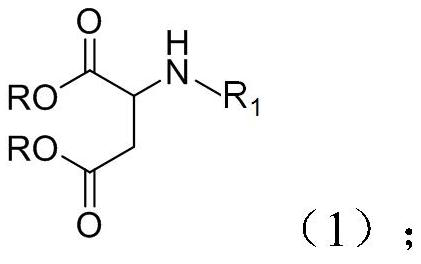
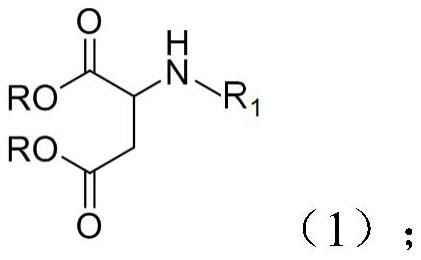
[0062] The second aspect of the present invention provides a method for preparing the above-mentioned polyurethane-aspartic polyurea resin, which comprises: reacting and drying an amino alcohol and a maleic acid diester to obtain the polyurethane-aspartic polyurea resin. The drying method may include drying under reduced pressure, drying under heating, and the like. The reaction of the present invention is carried out in an inert gas atmosphere, such as nitrogen, argon, etc., and the progress of the reaction can be monitored by TLC.
[0063] In one embodiment, the temperature of the reaction in the present invention is 60-100°C, which can be exemplified as 60°C, 65°C, 70°C, 75°C, 80°C, 85°C, 90°C, 95°C, 100°C °C.
[0064] A second aspect of the present invention provides a polyurethane-aspartic polyurea polymer, and the raw materials for preparing the polymer include the polyurethane-aspartic polyurea resin according to any one of claims 1 to 7, and a crosslinking agent.
[...
Embodiment 1~8
[0069] Embodiments 1 to 8 provide a polyurethane-aspartic polyurea resin
Embodiment 1
[0071] This example provides a polyurethane-aspartic polyurea resin. The raw materials for the preparation of the resin are amino alcohol and diethyl maleate, and the molar ratio is 1:2. The structural formula of the amino alcohol is as follows:
[0072] This example also provides the above-mentioned preparation method of the polyurethane-aspartic polyurea resin, including: adding 129 grams of amino alcohol into a four-necked bottle under the condition of nitrogen, then adding 344 grams of diethyl maleate, heating up To 80°C, the reaction endpoint was monitored by TLC. After the reaction was completed, excess diethyl maleate was removed under reduced pressure to obtain 288 g of polyurethane-aspartic polyurea resin with a yield of 95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com