Chiral nano vaccine as well as preparation method and application thereof
A nano-vaccine and chiral technology, which is applied in the field of chiral nano-vaccine and its preparation, can solve the problems of poor cellular immune response, low safety, and weak effective presentation of antigens, and achieve strong humoral immune effect and strong ability to adsorb antigens , The effect of promoting capture efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A hand -nano vaccine and its preparation methods include the following steps:
[0051] (1) Preparation of hand -hydrated nanoparticles: Soak the reaction bottle with king water for 24h, wash, and dry. Add 4ml of water to the reaction bottle, add 8mg / ml of potassium permanganate solution 1ml, 0.1ml oleic acid, 4.4 mg / ml of citrinine solution 0.3ml, 4mg / ml sodium hydroxide solution, 0.08ml of hydraulic acid, 0.08ml, and continuously stirring The color of the solution becomes black and stops stirring. Collect the reaction product 7000rpm centrifugal 5min, remove the upper clearing, sedimentation with pure water, repeatedly washing, and removing excess non -response.
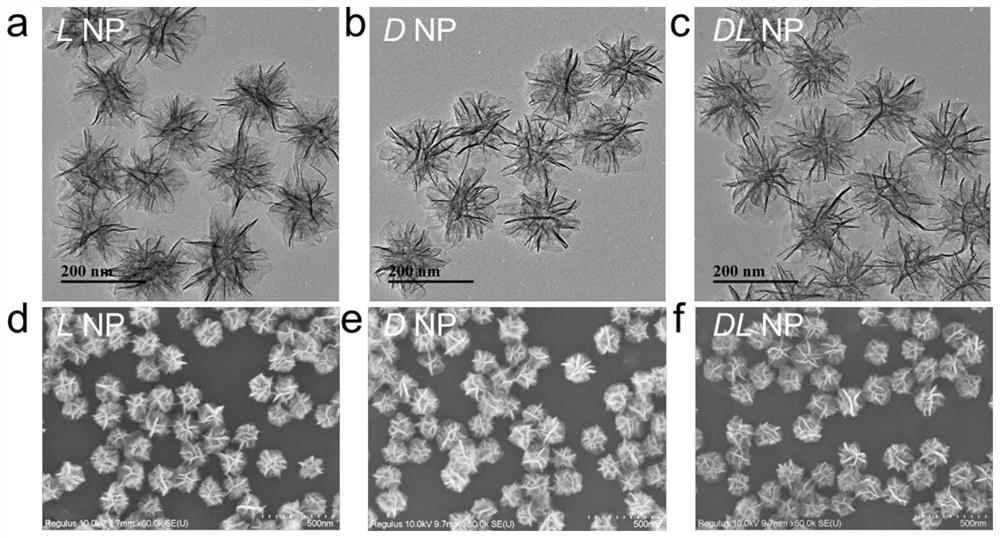
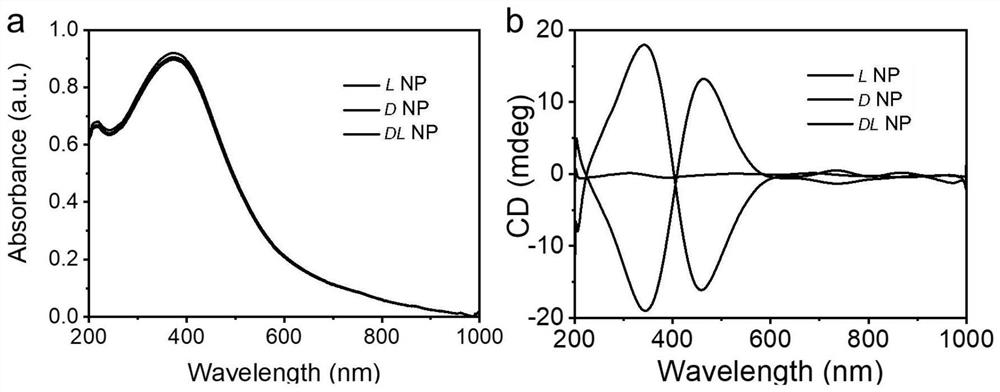
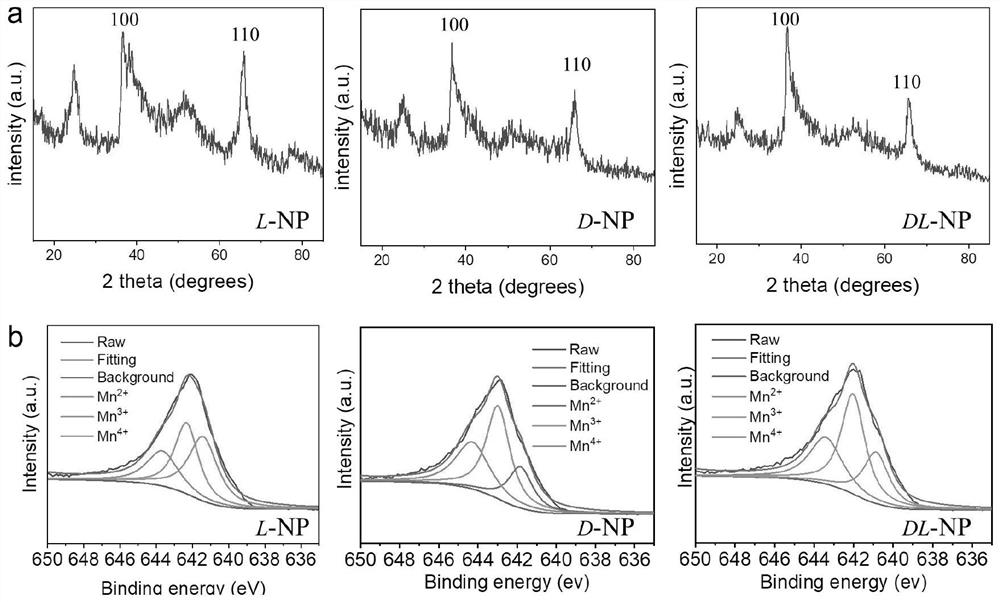
[0052] Example 1 The transmitted electron microscopy and scanning electron microscopic diagram of the hand -pre -manganese nanoparticles prepared by the Example 1 figure 1 Show, figure 1 A, 1B, 1C, which are transmitted electron microscopy for L, D, DL, manganese nanoparticles; figure 1 D, 1E, 1F, are scanned ele...
Embodiment 2
[0058] A hand -nano vaccine and its preparation methods include the following steps:
[0059] (1) Preparation of hand -hydrated nanoparticles: Soak the reaction bottle with king water for 24h, wash, and dry. Add 4ml of water to the reaction bottle, add 8mg / ml of potassium permanganate solution 1ml, 0.1ml oleic acid, 4.4 mg / ml of citrinine solution (L type or D) 0.3ml, 4mg / ml hydrogen oxidation The sodium solution is 0.08ml, continuously stirred until the color of the solution becomes black, and stops stirring. Collect the reaction product 7000rpm centrifugal 5min, remove the upper clearing, sedimentation with pure water, repeatedly washing, and removing excess non -response.
[0060] (2) Preparation of hand-manganese oxide nano-vaccine: Frozen the synthetic hand-manganese nanoparticles at -30 ° C and 8Pa. ), Stir 12h, 7000rpm centrifugal 5min, remove the upper clearing, you can get a hand -to -hand manganese nano -vaccine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com