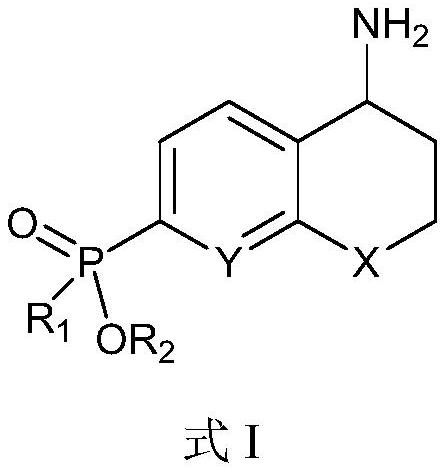
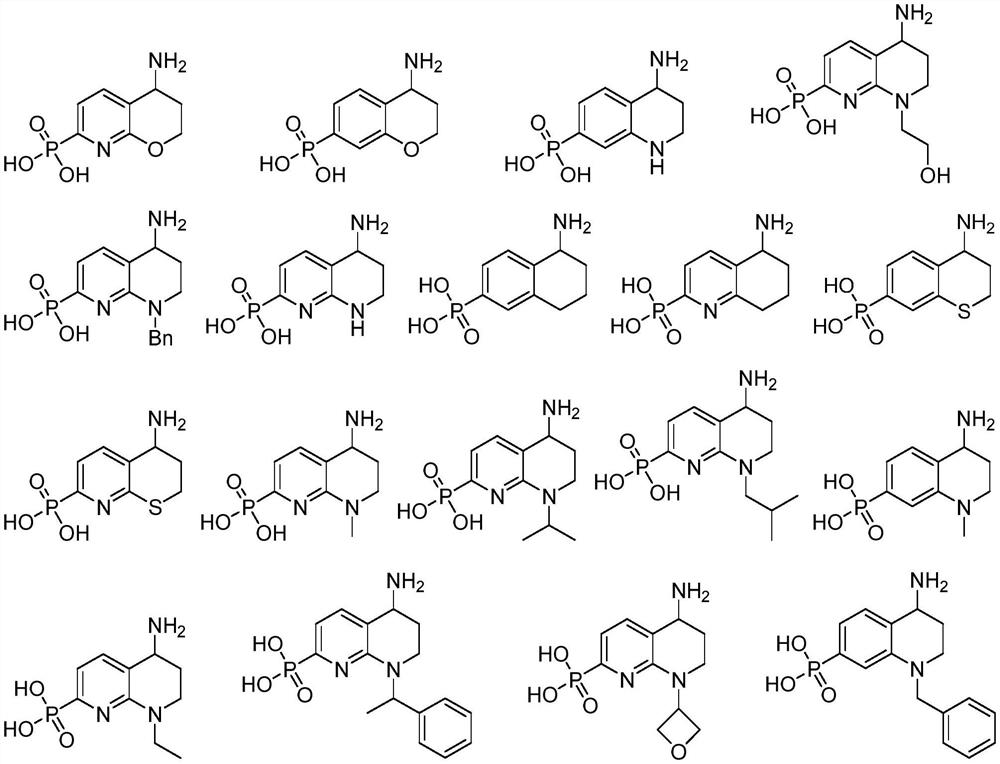
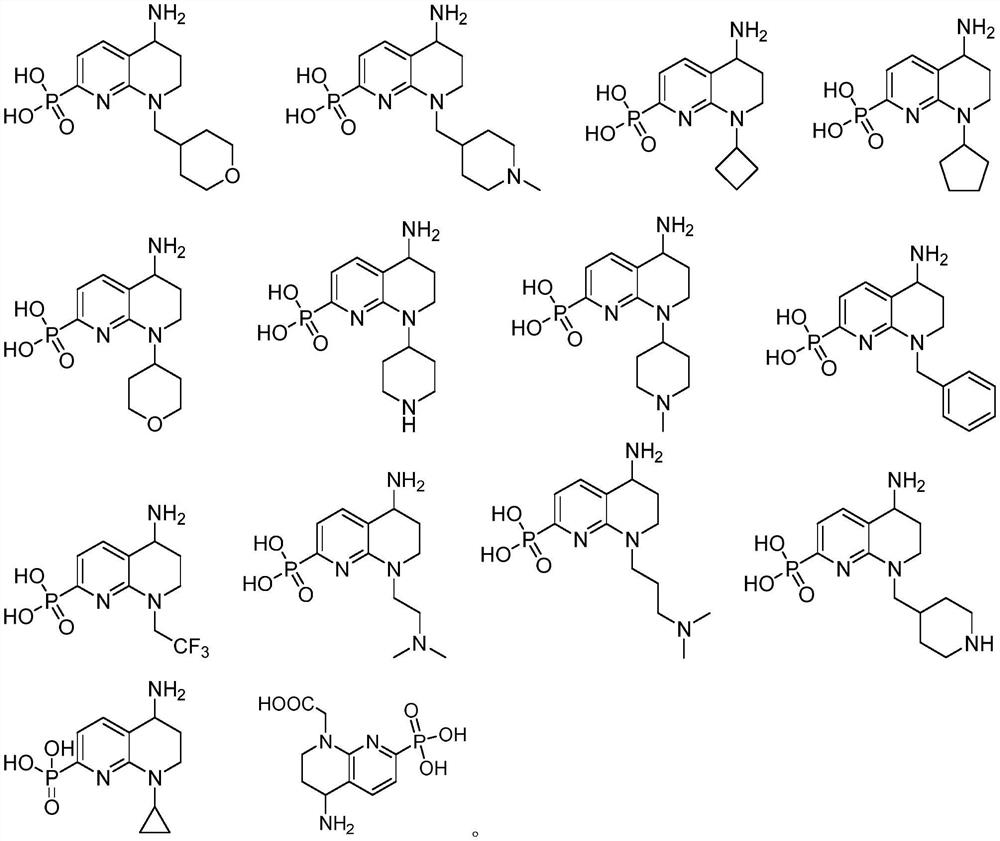
Plasmin inhibitor as well as preparation method and application thereof
An unsubstituted and selected technology, applied in the field of medicinal chemistry, can solve the problems of easily causing epilepsy, many adverse reactions, and large dosage of drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of (4-amino-3,4-dihydro-2H-pyrano[2,3-b]pyridin-7-yl)phosphonate hydrochloride
[0062]
[0063] Step 1: Preparation of (3-((tert-butyldimethylsilyl)oxy)propylene)-2-methylpropane-2-sulfinamide
[0064] Weigh 3-((tert-butyldimethylsilyl)oxy)propanal (5.6g), dissolve it in dichloromethane (50mL), add tert-butylsulfinamide (5.4g), anhydrous sulfuric acid Copper (23.8 g) was stirred at room temperature for 48 h. TLC showed that the starting material was consumed, filtered through celite and purified by column chromatography to give the title compound (7.35g).
[0065]
[0066] MS(ESI)m / z(M+H) + = 292.1.
[0067] Step 2: 3-((tert-Butyldimethylsilyl)oxy)-1-(2,6-dichloropyridin-3-yl)propyl)-2-methylpropane-2-sulfinyl Preparation of amides
[0068] Weigh 2,6-dichloropyridine (3.74g) into a 250mL three-necked flask, add tetrahydrofuran (50mL) to dissolve, replace with argon three times, cool to -78°C, and slowly add lithium diisopropylamide with...
Embodiment 2
[0088] Example 2 Preparation of (4-amino-3,4-dihydro-2H-benzopyran-7yl)phosphonate hydrochloride
[0089]
[0090] Step 1: Preparation of N-(7-bromobenzopyran-4-alkylene)-2-methylpropane-2-sulfinamide
[0091] 7-Bromo-4-dihydrochromone (2.5g) was dissolved in tetrahydrofuran (4mL), tert-butylsulfinamide (2.0g) and tetraethyl titanate (4mL) were added successively, and the reaction was carried out at 85°C overnight , TLC showed that the reaction of the starting material was complete. Water was added to quench, filtered, the filter cake was washed with ethyl acetate, the filtrate was backwashed twice with saturated brine (2×15 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain the title compound (2.1 g) ).
[0092]
[0093] MS(ESI)m / z(M+H) + = 329.9.
[0094] Step 2: Preparation of N-(7-bromobenzopyran-4-yl)-2-methylpropane-2-sulfinamide
[0095] Weigh N-(7-bromobenzopyran-4-alkylene)-2-methylpro...
Embodiment 3
[0107] Example 3 Preparation of (4-amino-1,2,3,4-tetrahydroquinolin-7-yl)phosphonate hydrochloride
[0108]
[0109] Step 1: Preparation of methyl 3-((3-bromophenyl)amino)propanoate
[0110] m-bromoaniline (9 g), methyl acrylate (4.6 g) and glacial acetic acid (297 μL) were placed in a sealed tube and reacted at 110° C. for 12 h. After TLC detected that the raw material was consumed, it was diluted with ethyl acetate, washed three times with water, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by column chromatography to give the title compound (10 g).
[0111]
[0112] MS(ESI)m / z(M+H) + = 257.9.
[0113] Step 2: Preparation of methyl 3-(N-(3-bromophenyl)-4-methylphenylsulfonamido)propanoate
[0114] Methyl 3-((3-bromophenyl)amino)propanoate (10 g) and 4-toluenesulfonyl chloride (8.1 g) were dissolved in pyridine (20 mL) and reacted at room temperature for 2 h. After TLC detected that the raw materials were consumed, the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com