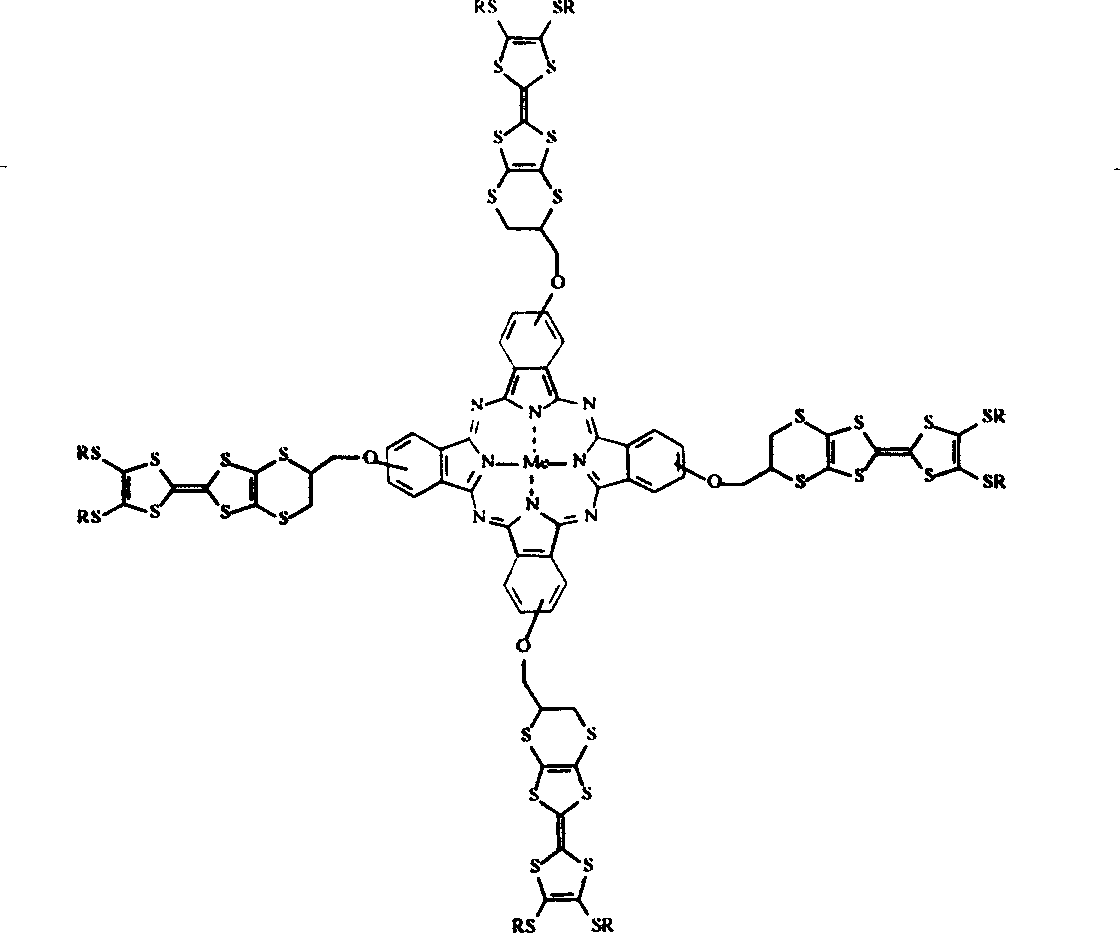
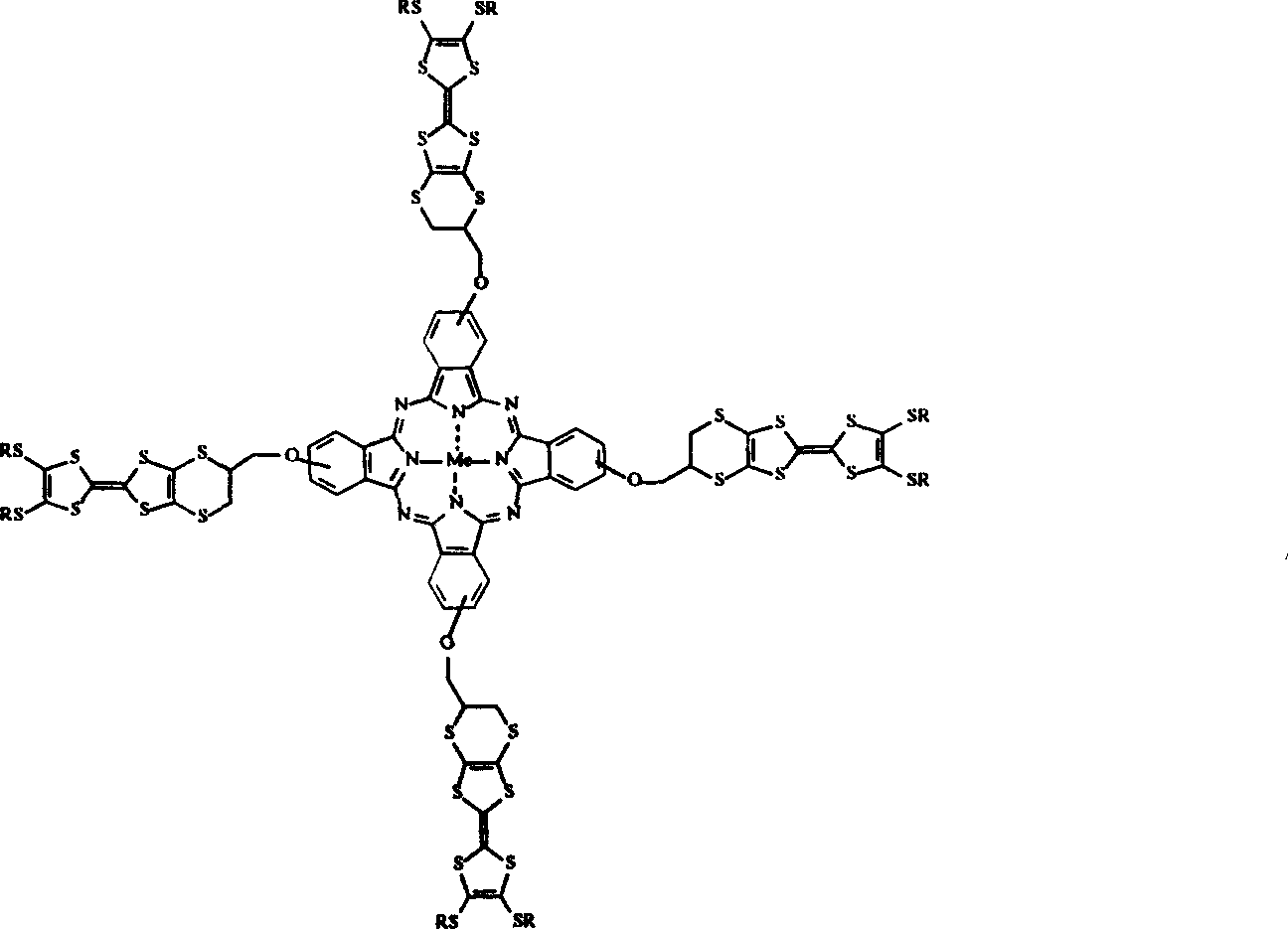
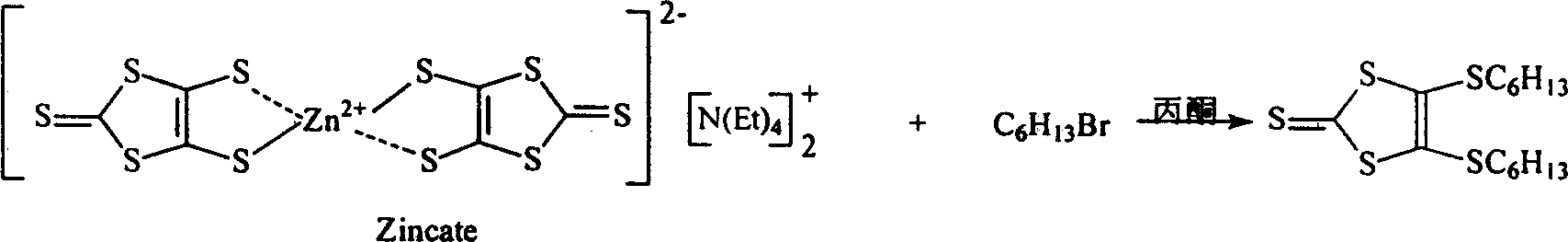Compound with intramolecule change transfer and its preparing process
A compound and molecular technology, applied in the field of compounds with intramolecular charge transfer and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of compound described in the present invention is described further below:
[0023] (1) 2-(4,5-Di-n-hexylthio-1,3-dithio-2-ylidene)-5-hydroxymethylene-5-hydrogen-6-dihydro-1,3-dithio Preparation of [4,5-b][1,4]dithiocyclohexane:
[0024] After dissolving zinc salt (Zincate) in acetone, add hexyl bromide dissolved in acetone dropwise in the boiling state, wherein the molar ratio of zinc salt (Zincate) to hexyl bromide is 1:5.3, continue to reflux for 2-4 hours, and filter with suction Remove insolubles. The solvent was distilled off, and the residue was purified by column chromatography to obtain 4,5-di-n-hexylthio-1,3-dithiol-2-thione, one of the raw materials required for the coupling reaction. The reaction formula is as follows: Show:
[0025]
[0026] After reflux for 20 hours in the same way, another raw material 5,6-dihydro-5-acetoxymethylene-1,3-dithio[4,5-b][1,4] was purified to obtain the coupling reaction Dithiocyclohexane-2-thione...
Embodiment
[0040] 4,5-Di-n-hexylthio-1,3-dithiole-2-thione
[0041] 8g zinc salt (Zincate) (11.2mmol) was dissolved in 100mL acetone, heated to reflux with stirring, 50mL acetone solution containing 9.4mL (60mmol) bromohexane was added dropwise, and refluxed for 4 hours. Suction filtration to remove insoluble matter. Acetone was removed by rotary evaporation. The residue was subjected to column chromatography (chloroform:petroleum ether=1:2 as the developing solvent) and the developing solvent was rotated to obtain 8 g of orange-red oil with a yield of 98%.
[0042] MS(EI): m / z(%)=367(M + +1, 14.119), 366 (M + , 27.196)
[0043] 5,6-Dihydro-5-acetoxymethylene-1,3-dithio[4,5-b][1,4]dithiocyclohexane-2-thione
[0044] Add 7.2g (10mmol) Zincate and 100mL acetone into a 250mL three-neck flask. 11.4 mL (80 mmol) 2,3-dibromopropyl acetate / 50 mL acetone were added dropwise under stirring and reflux, and the reaction was carried out for about 20 hours. TLC tracking, the developing solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com