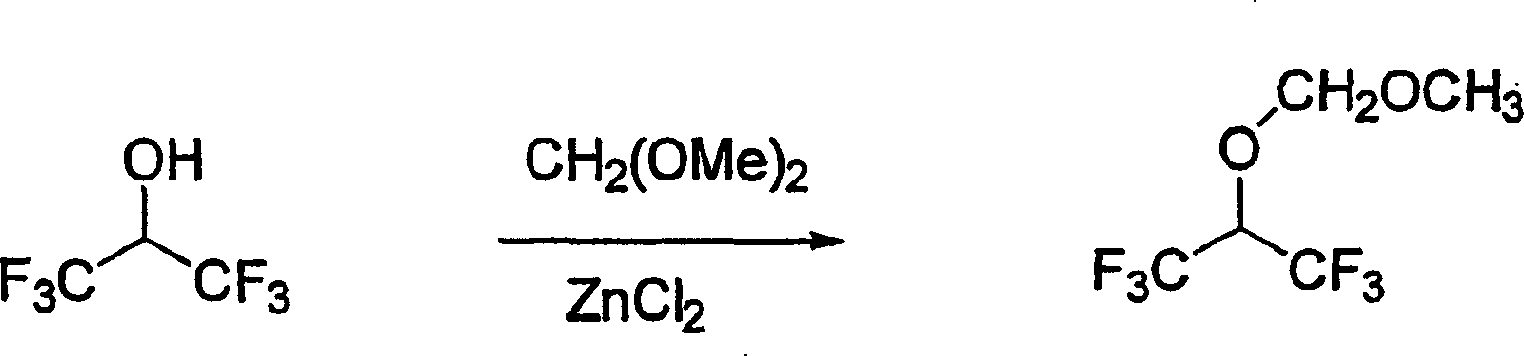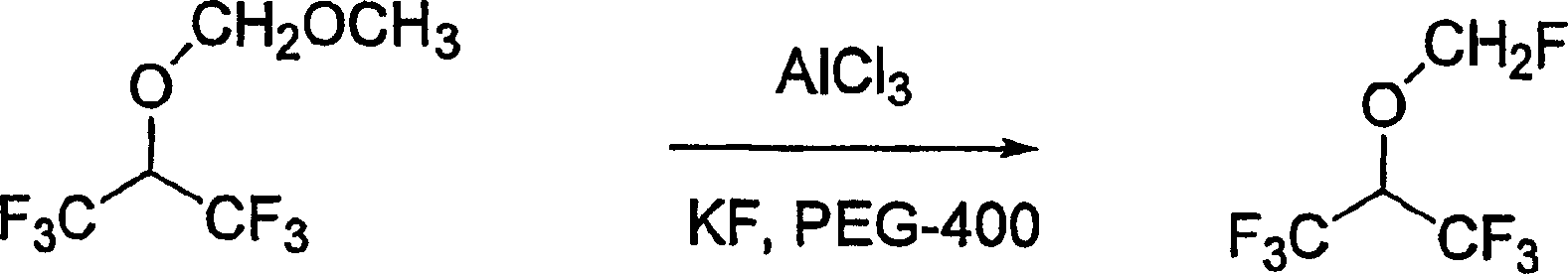Synthetic method for fluoromethylation of alcohols
A technology of fluoromethylation and halohydrin, which is applied in the field of fluoromethylation of halohydrin, and can solve problems such as expensive, low yield of fluorinated products, and influence on reversible regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] According to Reaction Scheme I, methoxy-1,1,1,3,3,3-hexafluoroisopropoxymethane was synthesized in the following manner.
[0038]
[0039] 1,1,1,3,3,3-methoxyl,1,1,3,3-
[0040] Hexafluoroisopropanol Hexafluoroisopropoxymethane
[0041] Reaction Scheme I
[0042] Add ice-cold and well-stirred ZnCl for 5 minutes 2 (41 g, 0.30 mol) in 1,1,1,3,3,3-hexafluoroisopropanol (31.5 mL, 0.31 mol) was slowly added dimethoxymethane (24 mL, 0.30 mol). The reaction mixture was allowed to warm to room temperature over 1 hour, then heated at reflux. After reflux for 6 hours, the contents of the reaction flask were distilled, leaving a solid residue in the flask. The distillate was washed with 2N NaOH (10×4), water (10 mL), brine (10 mL), the bottom organic layer was separated and dried over anhydrous sodium sulfate, filtered to give methoxy-1,1,1,3 , 3,3-Hexafluoroisopropoxymethane (34 g, 55%).
Embodiment 2
[0044] According to Reaction Scheme II, sevoflurane was synthesized in the following manner.
[0045]
[0046] Methoxy 1,1,1,3,3-
[0047] Sevoflurane
[0048] Hexafluoroisopropoxymethane
[0049] Reaction Scheme II
[0050] To methoxy-1,1,1,3,3,3-hexafluoroisopropoxymethane (3.57 g, 17 mmol) was added anhydrous AlCl at room temperature 3 (2.25 g, 17 mmol), then the reaction flask was heated at 95°C. After 14 hours, the reaction mixture was cooled to room temperature, then PEG-400 (5.0 mL) and KF (1.97 g, 34 mmol) were added. The reaction mixture was then reheated to 95°C. After 18 hours, the reaction mixture was cooled to room temperature and diluted with 20 mL of water. The lower organic layer was separated and distilled to give sevoflurane (2.4 g, 51%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com