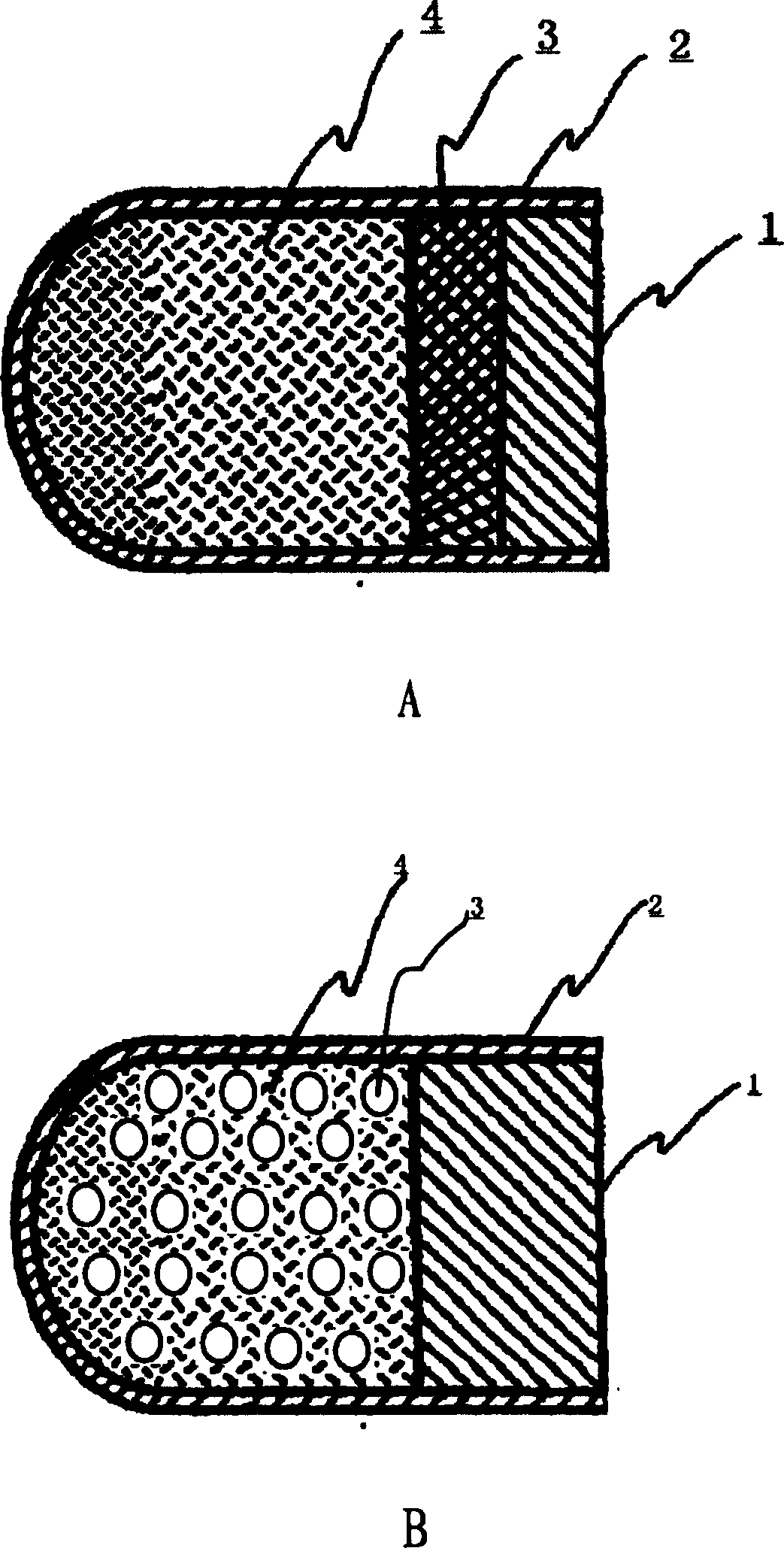
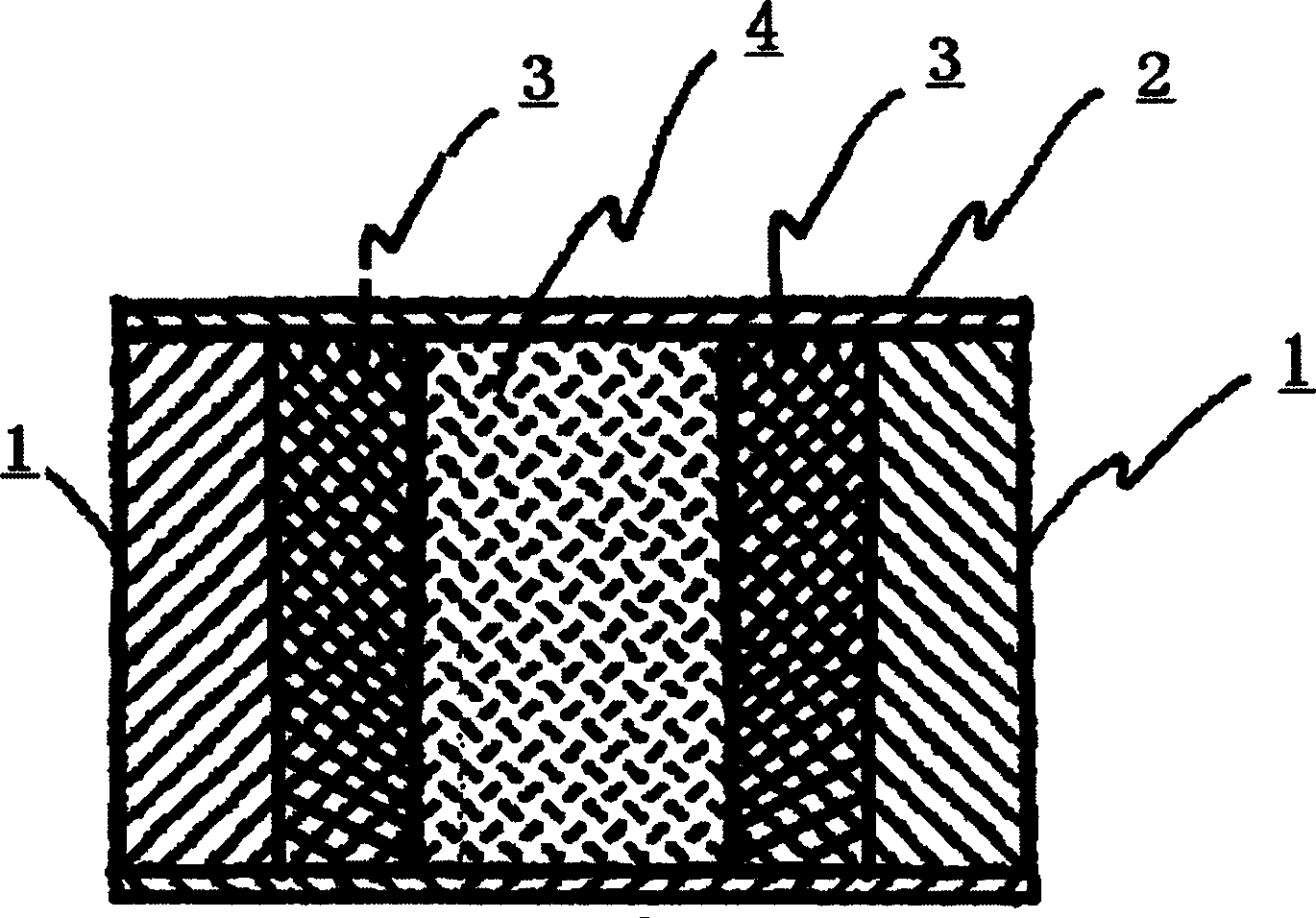
Floating type pulse release capsule in stomach
A technology of pulse release and gastric floating, which is applied in the field of medicine and can solve the problems of unguaranteed bioavailability of preparations and large differences in drug release time lag in vivo.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, Preparation of Verapamil Gastric Floating Pulse Release Capsules
[0026] a) Preparation of capsules
[0027] 11g of ethyl cellulose (EC) was dissolved in 100ml of dichloromethane solution, and 3ml of castor oil was used as a plasticizer to prepare an EC solution with a mass concentration of 11%, and then a capsule body was made in a No. 0 capsule mold.
[0028] b) Preparation of drugs
[0029] After mixing 40g verapamil with 10g lactose and 15g sodium carboxymethyl starch auxiliary materials, use 5% polyvinylpyrrolidone ethanol solution as a binding agent to make a soft material, pass through a 16-mesh sieve for granulation, and dry at 55°C for 45 Minutes, sieve and granulate, add 0.5g magnesium stearate and mix well, punch with a standard diameter of 6 mm, press each tablet containing 40mg verapamil, a total of 1000 tablets.
[0030] c) Preparation of Capsule Plugs
[0031] Mix 60mg of low-viscosity hydroxypropyl methylcellulose (HPMC) with 40mg of lacto...
Embodiment 2
[0033] Embodiment 2, preparation of the capsule plug of gastric floating pulse release capsule
[0034] According to the preparation method of embodiment 1c) capsule plug, prepare erodible capsule plug with hydroxypropyl methylcellulose HPMC-E5 and hydroxypropyl methylcellulose HPMC-E50 and lactose respectively, and each weight is 100mg, and its composition And the control time (delay) is as follows:
[0035] serial number
Embodiment 3
[0036] Embodiment 3, capsule pulse release test of the present invention
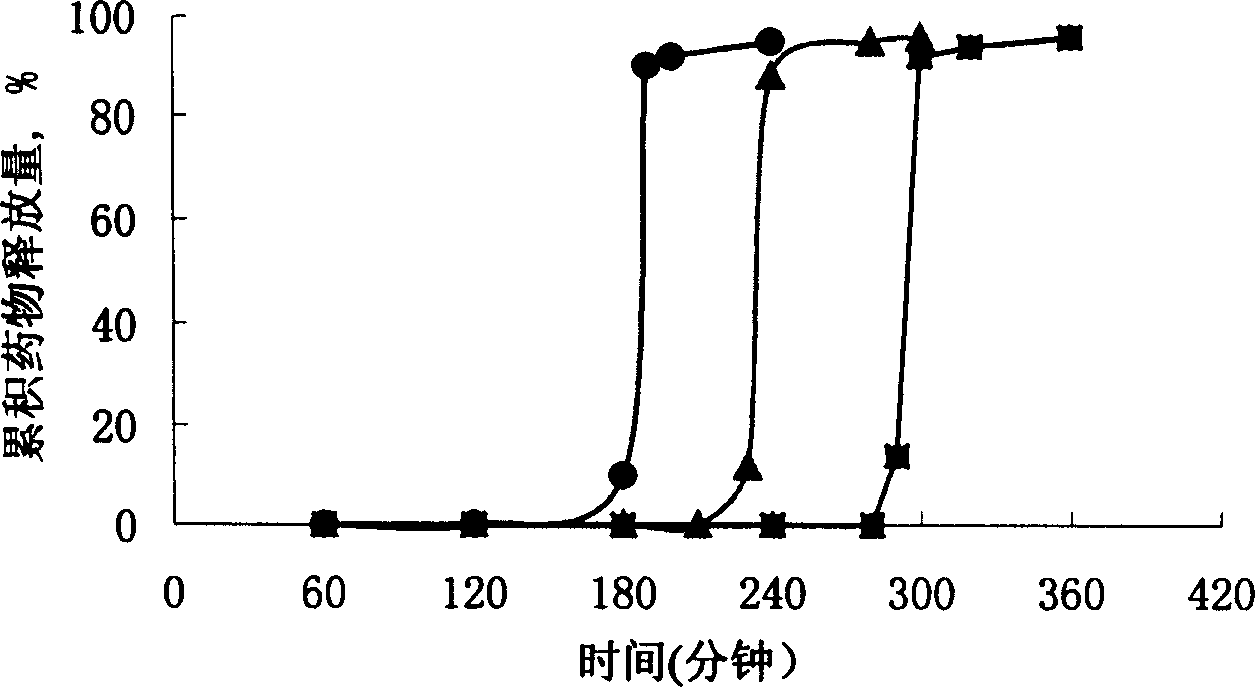
[0037] For the three capsule plugs numbered 4, 5, and 6 in Table 1, gastric floating pulse-release capsules were prepared according to the preparation method of Example 1. Carry out release test according to "Chinese Pharmacopoeia" 2000 edition appendix XC dissolution test method second method paddle method, release condition: take 900ml artificial gastric juice as release medium, rotating speed (100 ± 1) r / min, temperature (37 ± 0.5) ℃. Put the capsules into the settling basket, sink to the bottom of the container, turn on the rotary switch, and time. Regularly sample 6ml, filter with a 0.8 μm microporous membrane, and supplement with the same amount of dissolution medium. After the filtrate is diluted, measure the absorbance at 229 nm, calculate the medium concentration according to the standard curve, and further calculate the dissolution percentage. release curve as image 3 As shown, ● is number...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com