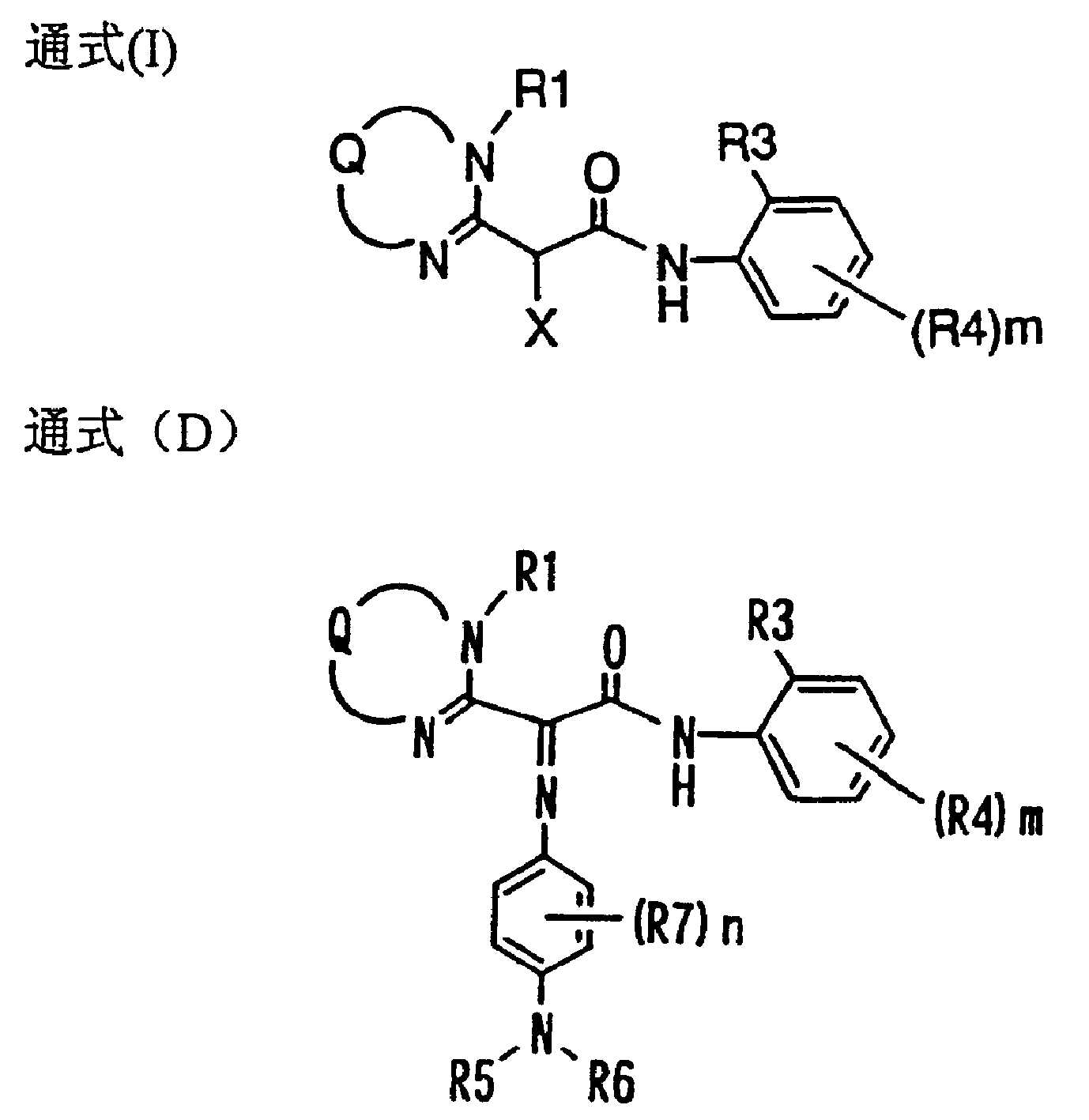
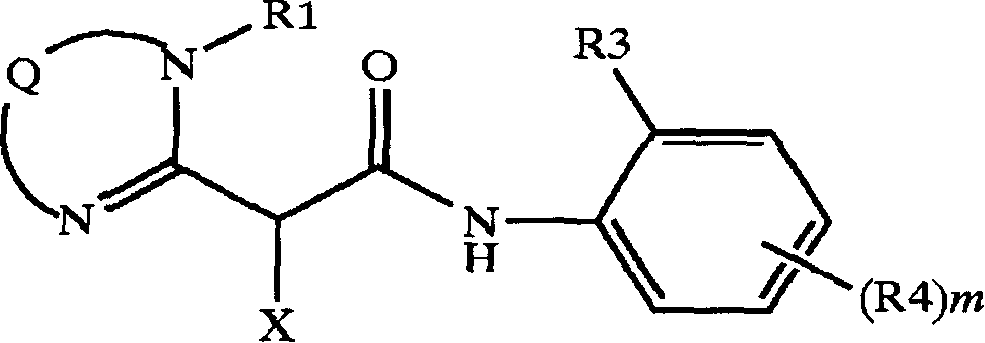
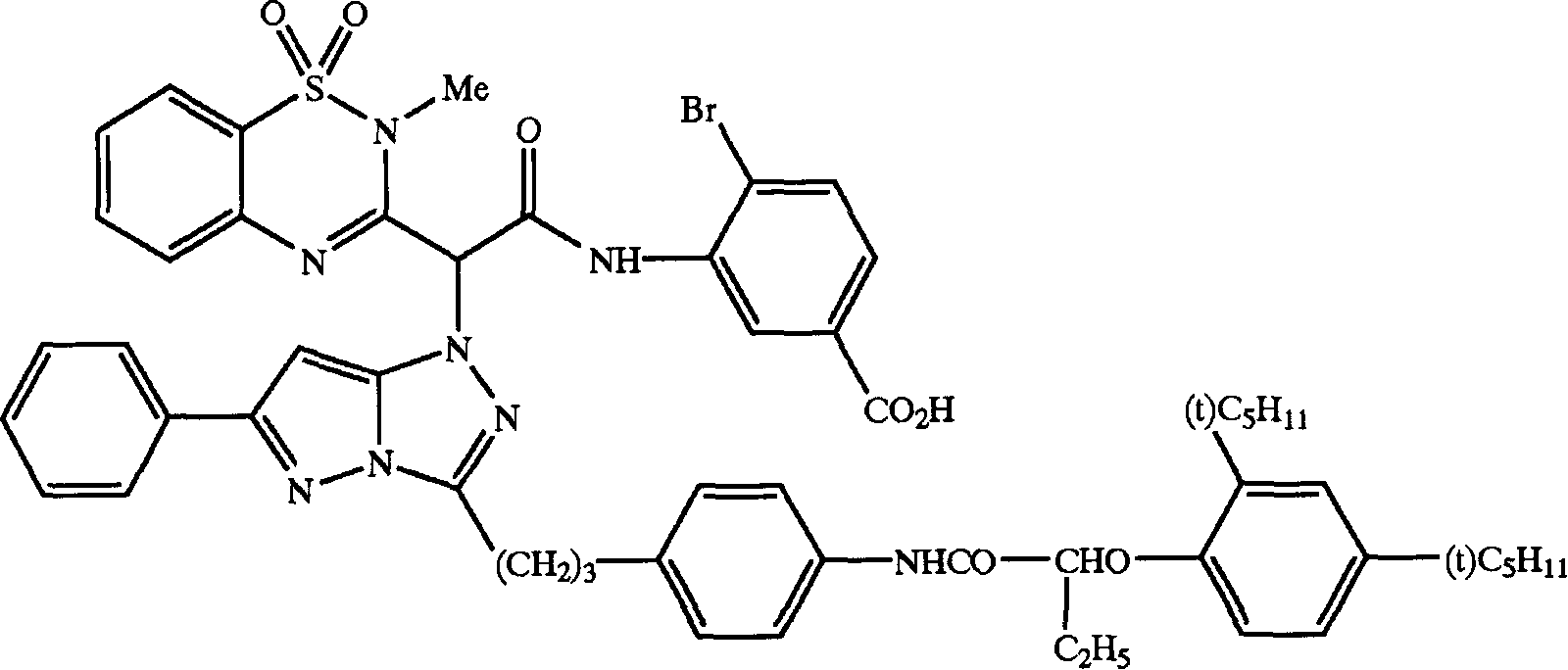
Color coupler for forming dye, silver halide photosensitive material and azomethine dyes compounds
A dye compound and color coupler technology, which is applied in the direction of azomethylamine dyes, organic dyes, azo dyes, etc., can solve problems such as insufficient image stability and vividness, reduced vividness, and blurred image outlines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0205] Synthesis Example 1: Synthesis of Coupler-(1)
[0206] Coupler-(1) was synthesized according to the following route.
[0207]
[0208] Coupler (1)
[0209] To 38.8 g of 40% aqueous methylamine solution and 200 ml of acetonitrile solution, a total of 44.3 g of o-nitrobenzenesulfonyl chloride was added a little at a time while stirring in an ice bath. The temperature of the reaction system was raised to room temperature, and stirred for another 1 hour. Ethyl acetate and water were added for liquid separation, and the organic layer was washed with dilute hydrochloric acid and saturated brine. After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off in vacuo to obtain 28.6 g of compound (A-1) crystallized from a mixed solvent of ethyl acetate and n-hexane.
[0210] Disperse 44.8g of reduced iron and 4.5g of ammonium chloride into 270ml of isopropanol and 45ml of water...
Synthetic example 2
[0215] Synthesis Example 2: Synthesis of Coupler-(3)
[0216] Coupler-(3) was synthesized according to the following route.
[0217]
[0218] Coupler(3)
[0219] To a solution of 438 g of 3-(2,4-di-tert-pentylphenoxy)propylamine, 210 ml of triethylamine, and 1 liter of acetonitrile, add a total of 333 g of o-nitrobenzenesulfonate in small amounts while stirring under an ice bath acid chloride. The temperature of the reaction system was raised to room temperature, and stirred for another 1 hour. Ethyl acetate and water were added for liquid separation, and the organic layer was washed with dilute hydrochloric acid and saturated brine. After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off in vacuo to obtain 588 g of compound (B-1) crystallized from a mixed solvent of ethyl acetate and n-hexane.
[0220] Disperse 84.0 g of reduced iron and 8.4 g of ammonium chloride into ...
Synthetic example 3
[0225] Synthesis Example 3: Synthesis of Coupler-(6)
[0226] Coupler-(6) was synthesized according to the following route.
[0227]
[0228] Coupler(6)
[0229] To a solution of 21.4 g of dibenzamide and 200 ml of acetonitrile, a total of 39.9 g of o-nitrobenzenesulfonyl chloride was added in small amounts while stirring in an ice bath. The temperature of the reaction system was raised to room temperature, further 30 ml of triethylamine was added dropwise, and stirred for 1 hour. Ethyl acetate and water were added for liquid separation, and the organic layer was washed with dilute hydrochloric acid and saturated brine. After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off in vacuo to obtain 31.2 g of compound (C-1) crystallized from a mixed solvent of ethyl acetate and n-hexane.
[0230] Disperse 44.8g of reduced iron and 4.5g of ammonium chloride into 270ml of isopropanol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com