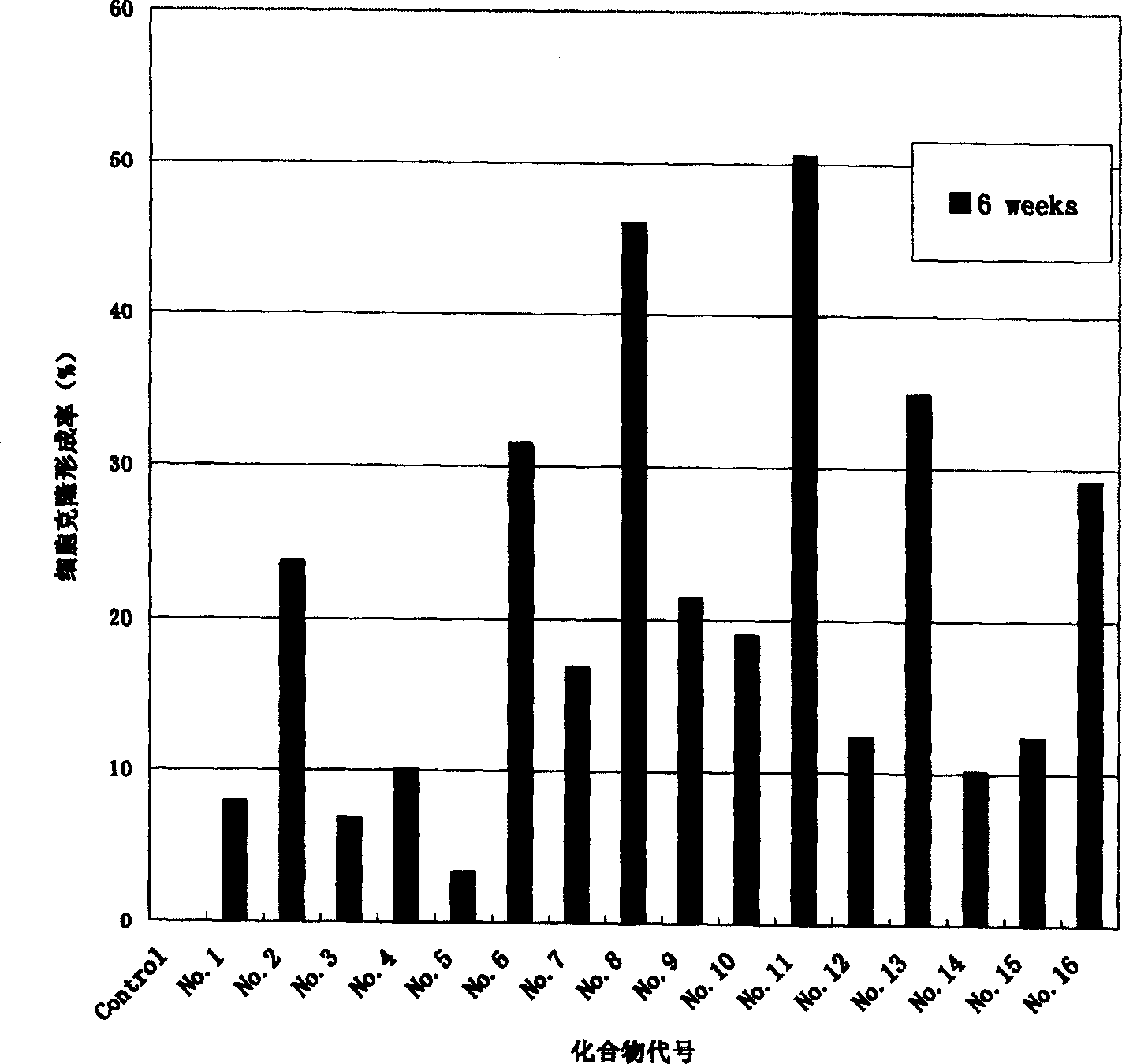
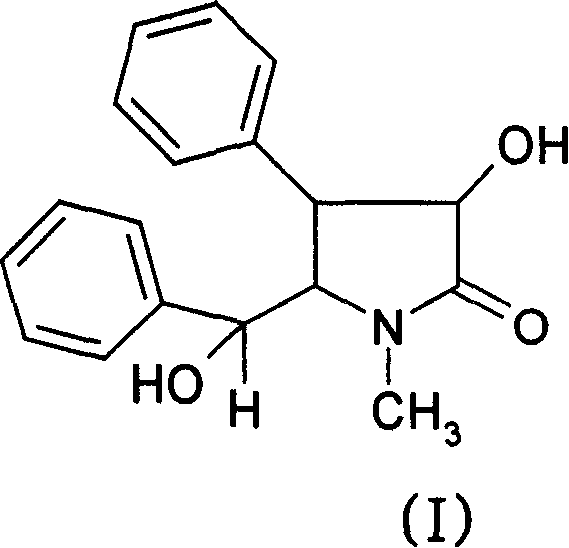
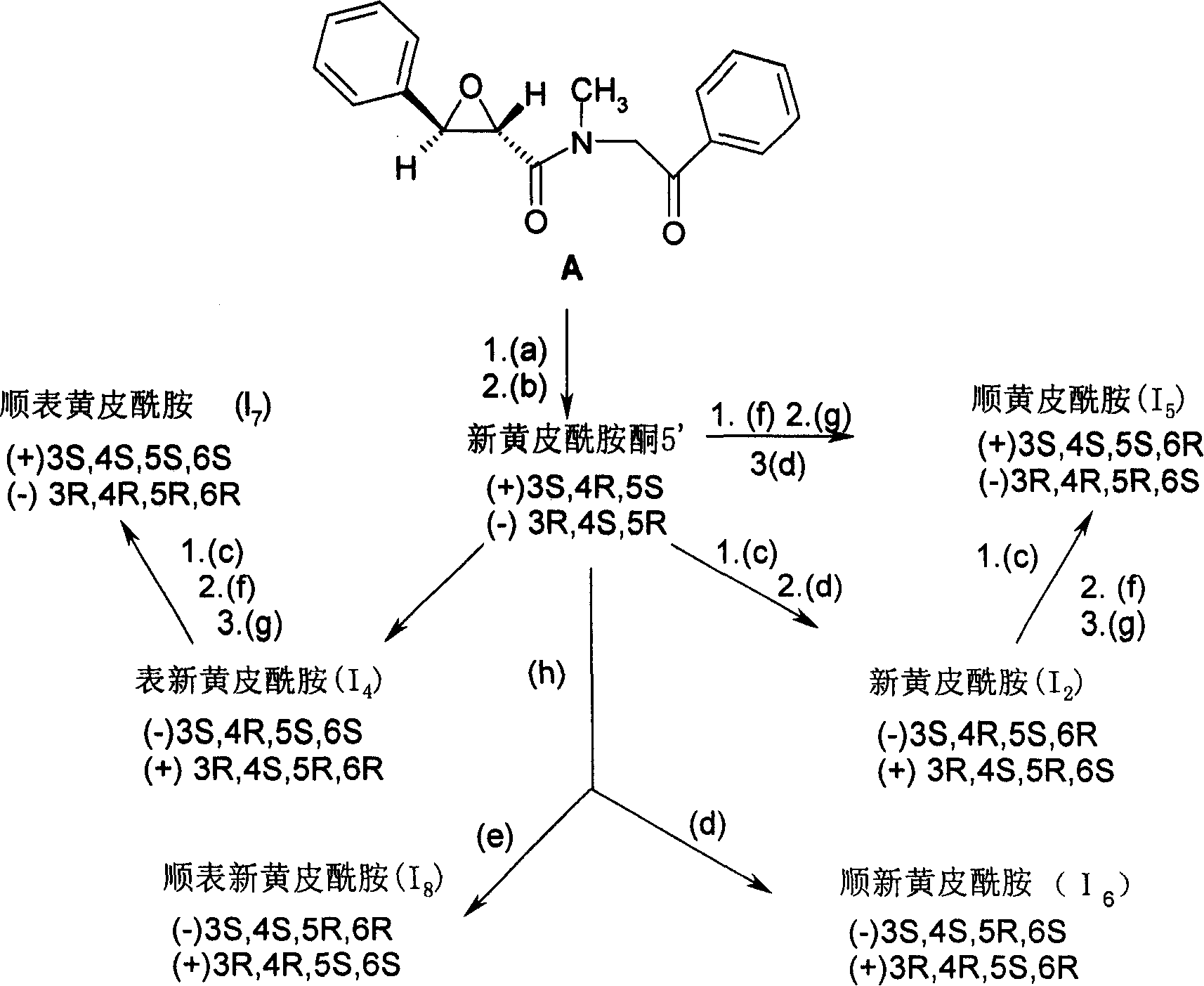
New Optical active derivative of flavo acidamide, its preparation method and its medicinal composition and use
A kind of xanthoamide, photoactive technology, applied in the direction of drug combination, organic chemistry, etc., can solve the problem that can not be directly used to prepare xanthanamide and its derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1 photoactive new xanthamide (I 2 ) preparation (see route 1)
[0068] Separation of racemic neobantamide ketone:
[0069] Add 2.7 g of racemic neopantyl ketone to 30 mL of dichloromethane solution of (-)-menthol oxyacetyl chloride prepared from 2.7 g of (-)-menthol oxyacetic acid, cool in an ice-water bath, and then add 1.5 mL of pyridine, stirred at room temperature until the reaction was complete, the reaction solution was diluted with 50 mL of dichloromethane, washed with 2N hydrochloric acid, saturated sodium carbonate solution and brine successively, and the organic layer was dried over anhydrous sodium sulfate. Concentrate to obtain 6g of oily matter, add hexane to solidify, filter the solid, and recrystallize from methanol to obtain 1.48g of 3-O-[(-)mentholoxyacetyl]--(3R,4S,5R)-neopanthamide Ketone [ester (a)], mp: 170-172°C, [α] D 15 =-50.9(c, 1.25, CHCl 3 ), yield 33.8%. The column chromatography of the filtrate gave 3-O-[(-)menthol oxyacetyl...
Embodiment 2
[0073] Embodiment 2 light active table new xanthamide (I 4 ) preparation
[0074] (+)-(3S, 4R, 5S)-Neospermide ketone 1.05g (3.56mmol.), aluminum isopropoxide 2.4g (11.75mmol.) dissolved in 30ml of anhydrous anhydrous isopropanol, heated and stirred , slowly evaporate isopropanol and acetone, and at the same time add anhydrous isopropanol dropwise at a constant speed, the reaction is completed in about 6.5 hours, evaporate most of the solvent, cool to room temperature, add 60ml water and 20ml 3N hydrochloric acid and stir at room temperature, a large amount of White solid, recrystallized from methanol to obtain (-)-(3S, 4R, 5S, 6S)-epipanthamide, yield 85%, m.p.217-219℃, [α] D 24 = -36.5 (c, 0.128, MeOH). Recrystallized again from methanol, m.p. 221-222°C, [α] D 20 = -37.3 (c, 0.15, MeOH).
[0075] (-)-(3R, 4S, 5R)-neopsinamide ketone is used as raw material, the same operation as above, and reduction with aluminum isopropoxide, to obtain (+)-(3R, 4S, 5R, 6R)-epideneopsi...
Embodiment 3
[0076] Embodiment 3 epibantamide (I 3 ) preparation (see route 2)
[0077] A: (±)-Epidermide ((±)-I 3 ) preparation
[0078] (±)-(4R*, 5R*, 6R*)-3-deoxyepixanthamide (3-deoxyl 3 )90mg (0.032mmol.), dissolved with 2.5mL anhydrous THF and 0.7mL hexamethylphosphoramide (N 2 under protection), cool to -70°C, stir for 5 minutes, add 1mL (0.32mmol.) fresh THF solution of lithium diisopropylamide with a syringe, stir for 1 hour at -60°C to -70°C, add triphosphite Ethyl ester 53ul, pass into O 2 , keep the temperature at -60°C to -70°C, stop ventilation after 2 hours, adjust the reaction solution to pH value = 3-4 with 0.5mol / L hydrochloric acid in an ice-water bath, then wash with ethyl acetate, water, and chlorination Wash with sodium aqueous solution, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain 40 mg of the title compound by column chromatography. The recovered raw material is 40 mg, with a yield of 40%. Recrystallization mp: 193-195°C.
[0079] B: (-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com