Combination of a nucleic acid and a vasoactive agent for enhanced gene delivery
An angiogenesis and blood vessel technology, applied in vector-mediated gene delivery, adenovirus constructs, treatment of peripheral vascular disease and heart disease, technology for delivering required genes, vector constructs, can solve systemic expression of transgenes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
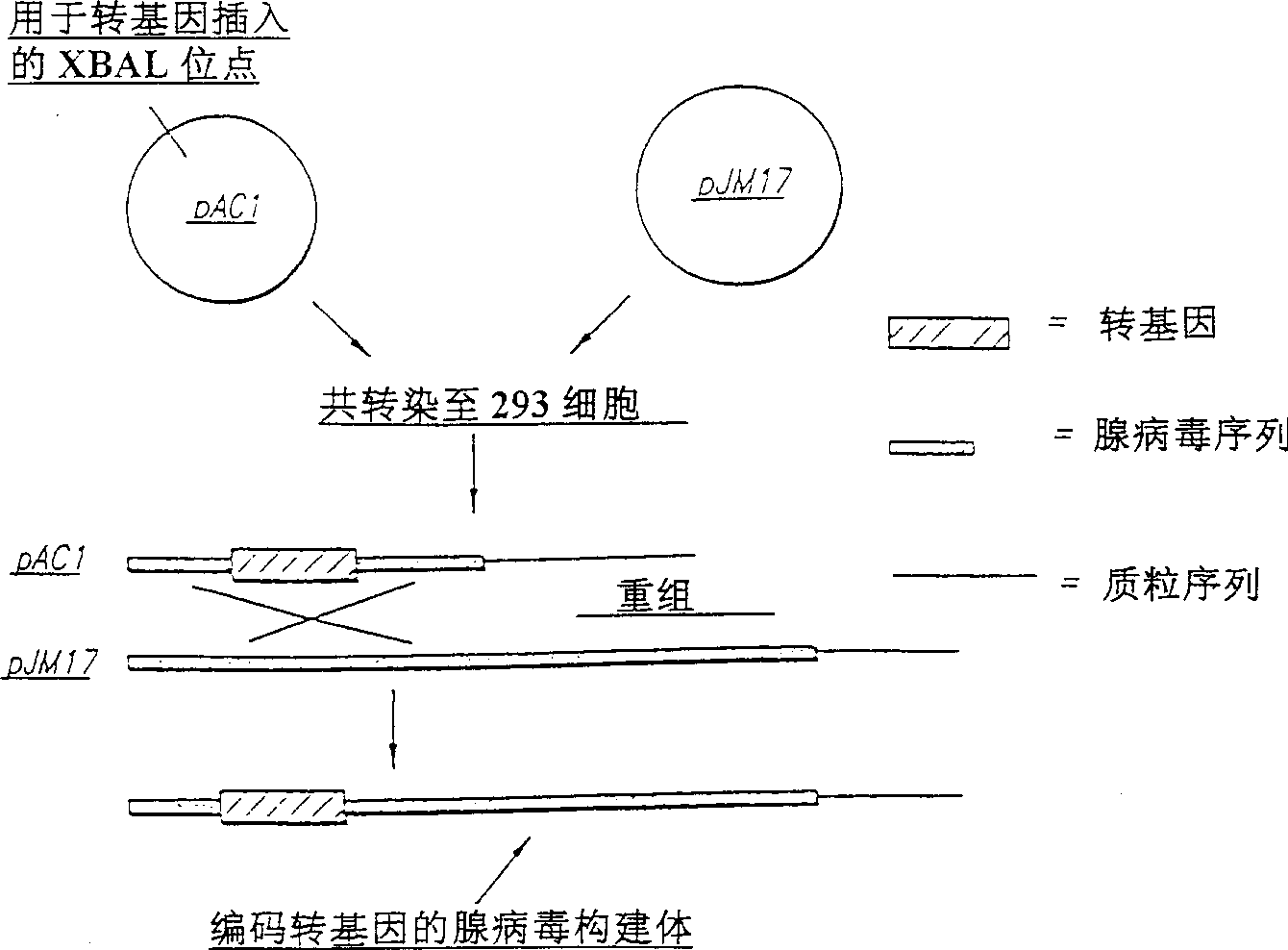
[0057] To facilitate the understanding of the invention, the following examples are provided to describe the results of a series of experiments. Of course, the experiments related to the present invention should not be regarded as particularly limiting the present invention, and modifications to the present invention, now known or later produced, are readily made by those skilled in the art, and fall within the scope of the invention described herein and hereinafter required. within the scope of the present invention. Example 1: Adenovirus constructs
[0058] A helper-independent replication-deficient human adenovirus 5 system was used. The target genes are lacZ and FGF-5. Release the full-length cDNA of human FGF-5 from the plasmid pLTRI22E (Zhen et al., Molecular Cell Biology, 8:3487,1988) in the form of a 1.1kb EcoRI fragment, which includes the gene open reading frame of 981bp, and clones the fragment into In the polylinker of plasmid ACCMVPLPA, which contains the CMV p...
Embodiment 2
[0060] Adult rat cardiomyocytes were prepared by Langendorf perfusion with collagenase-containing perfusate according to standard methods. The rod-shaped cells were cultured on a culture plate covered with laminin, and after 24 hours, the cells were infected with the adenovirus encoding β-galactosidase obtained in Example 1, and the multiplicity of infection was 1:1. After an additional 36 hours, cells were fixed with glutaraldehyde and incubated with X-gal. Following infection with recombinant adenovirus, typically 70-90% of adult cardiomyocytes express the β-galactosidase transgene. No cytotoxicity was observed when the MOI was 1-2:1.
Embodiment 3
[0061] Example 3: Myocardium in pigs
[0062] According to the method of Example 1, the adenovirus vector encoding β-galactosidase obtained in Example 1 was propagated in 293 permissive cells, and purified by CsCl gradient ultracentrifugation so that the final virus titer was 1.5×10 10 virus particles. Anesthetized and ventilated 40kg pigs were subjected to thoracotomy, a 26-gauge butterfly needle was inserted into the middle of the left anterior descending (LAD) coronary artery, and 2ml of vehicle (1.5×10 10virus particles). Suture the chest and allow the animal to recover. On day 4 post-injection, animals were sacrificed. Hearts were fixed with glutaraldehyde, sectioned and incubated with X-gal for 16.5 hours. After embedding and sectioning, tissues were counterstained with eosin.
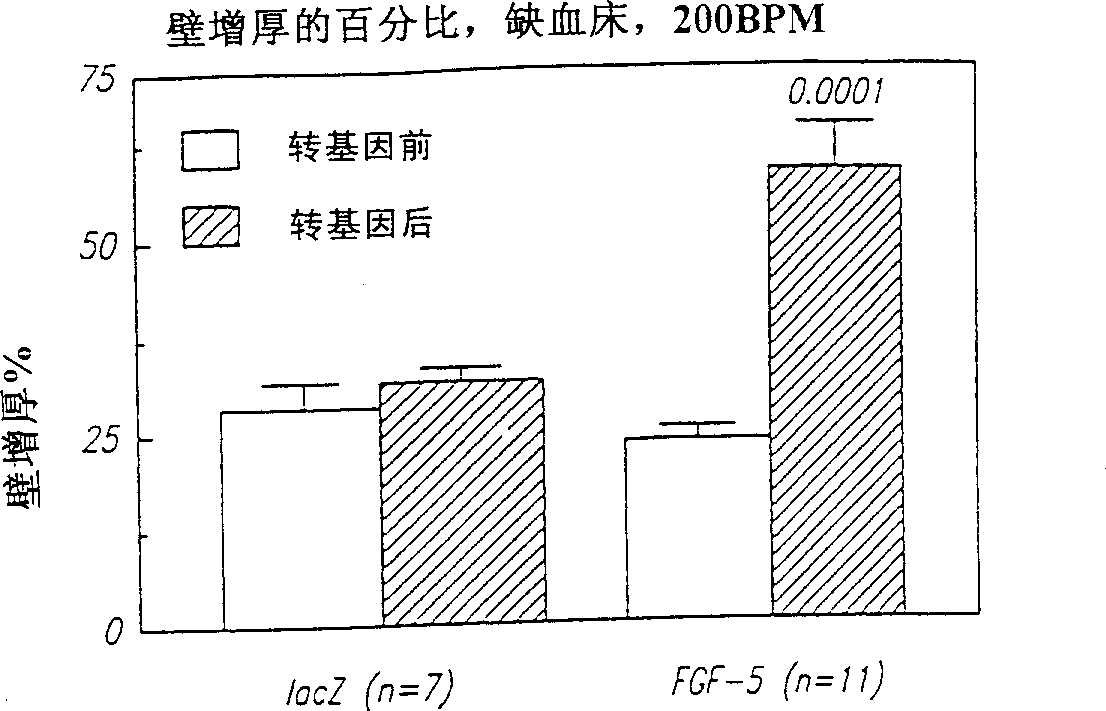
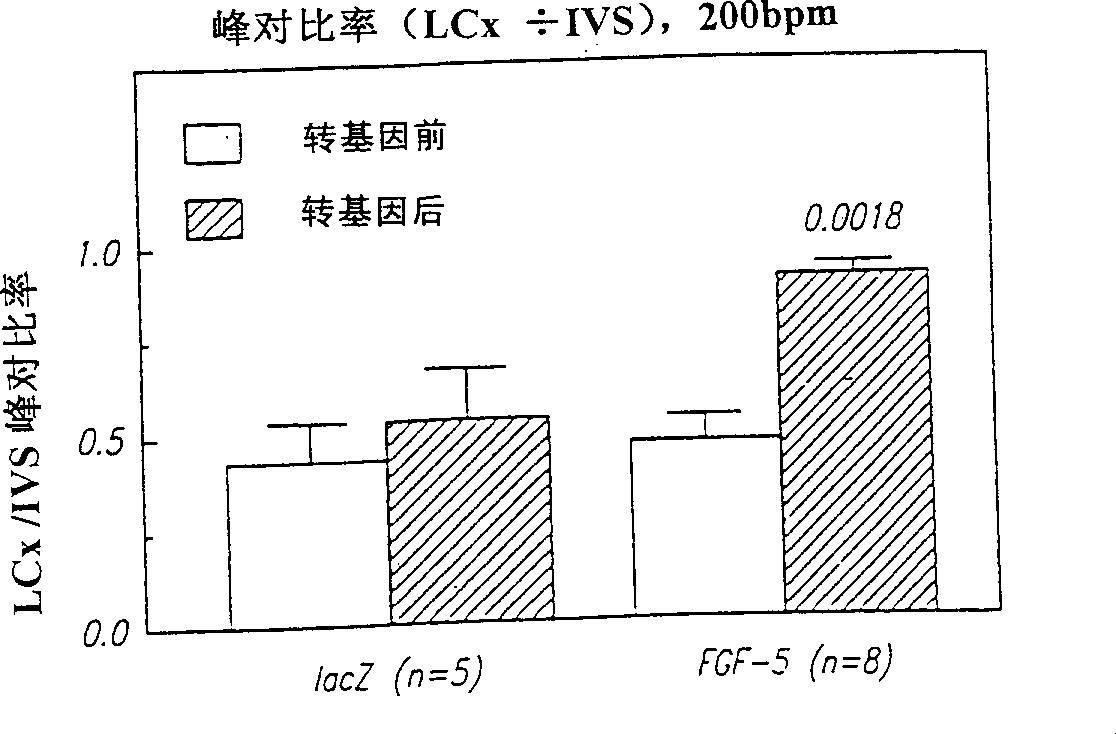
[0063] Microscopic analysis of tissue sections (transmural sections of the LAD bed 96 hours after intracoronary injection of adenovirus containing lacZ) showed that substantial gene transfer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com