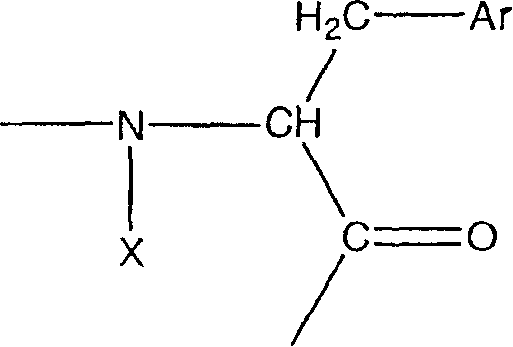
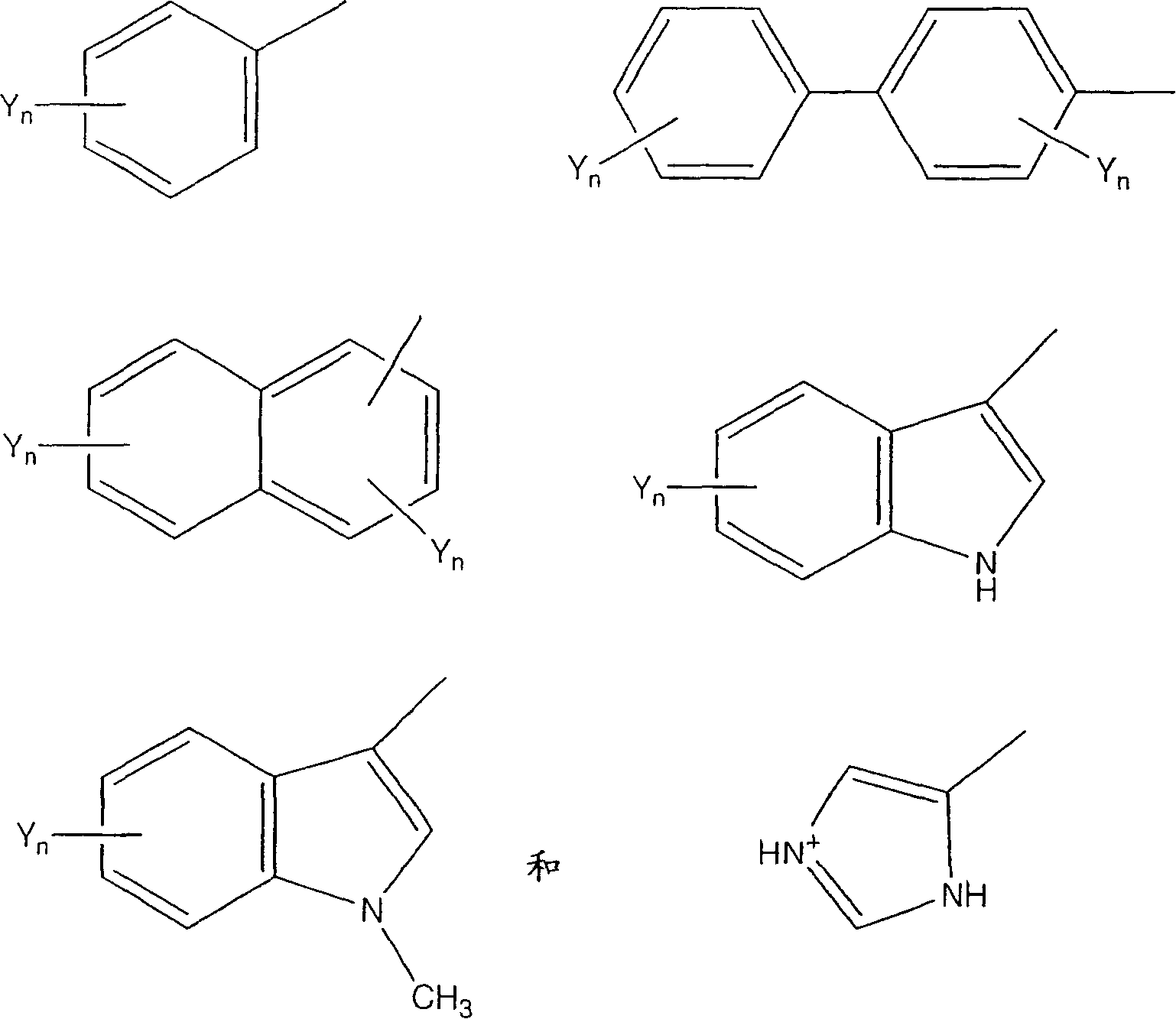
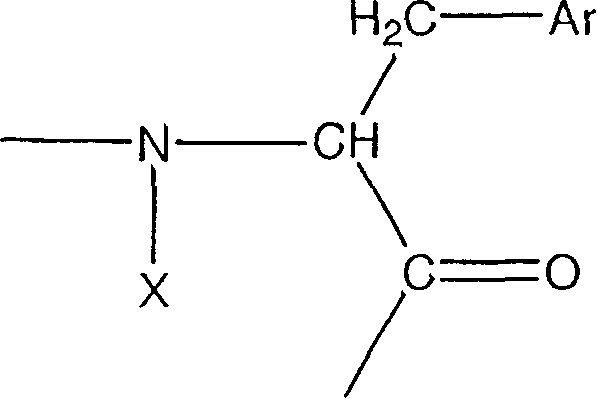
Urotensin-II agonists and antagonists
A technology of urotensin and isomers, applied in the field of urotensin-II polypeptide agonists and antagonists, can solve the problem that the receptor is not fully characterized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys)-Cpa-amide
[0050] Step 1: Preparation of Boc-4-chlorophenylalanine-S-methylbenzyl-D-cysteine-3-pyridyl-2-alanine-D-tryptophan-N 8 -Benzyloxycarbonyl-lysine-valine-S-methylbenzyl-cysteine-4-chlorophenylalanine-benzhydrylamine resin.
[0051] Diphenylmethylamine-polystyrene resin (Advanced ChemTech, Inc., Louisville, KY) (1.2g, 0.5mmol) was placed in the reaction vessel Advanced ChemTechpeptide synthesizer (Model 200) in the form of chloride ions, and the following procedures were carried out: Said reaction cycle: (a) methylene chloride; (b) 33% trifluoroacetic acid in methylene chloride (1 minute 2 times, each 25 minutes); (c) methylene chloride; ( d) ethanol; (e) methylene chloride; (f) 10% triethylamine in chloroform.
[0052] Described neutralization resin and Boc-4-chlorophenylalanine and diisopropylcarbodiimide (each 1.5mmole) in methylene chloride 1h, the aminoacid resin of gained is in the above-me...
Embodiment 2
[0056] Example 2: Analysis of U-II Antagonists Using Rat Aortic Ring Dissection
[0057] Male Sprague-Dawley rats (250-350 g) isolated 5-7 days prior to the experiment were sacrificed by decapitation (experiment approved by the Advisory Committee For Animal Resources, Tulane University School of Medicine). The thoracic aorta was dissected, freed from the associated tissue, and cut into approximately 1.5 mm wide rings. Said ring was suspended in 15ml containing high potassium Kreb's solution (9.15g / L potassium chloride, 2.1g / L sodium bicarbonate, 1.0g / L glucose, 0.16g / L monobasic potassium phosphate, 0.14g / L magnesium sulfate (dehydrated), and 0.22 g / L calcium chloride (dihydrated)) in organ baths.
[0058] Optimal tension (0.2 g) was applied to the tissue, and the bath medium was maintained at 37°C and filled with 95% O 2 / 5%CO 2 Mix and bring to a boil. Before fixing the organ bath, the selected preparation was swabbed with a damp cotton wool swab to remove the endothelia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com