Methods instruments and materials for chondrocyte cell transplantation
一种软骨细胞、软骨的技术,应用在软骨细胞移植领域,能够解决缺乏功能性组织修复生物力学特性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] in CO 2 Chondrocytes were cultured in the above growth medium for three weeks at 37°C in an incubator and operated in a class 100 laboratory at Verigen Transplantation Service ApS, Copenhagen, Denmark or Lübeck University, Lübeck, Germany. [Note: Compositions of other growth media can also be used to culture chondrocytes. ] Cells were trypsinized with EDTA for 5 to 10 minutes and counted by trypan blue viability staining in a Bürker-Türk chamber. Adjust the cell number to 7.5 x 10 per mL 5 cartilage cells. Place a piece of NUNCLON in said level 100 lab TM Plate open the cover.
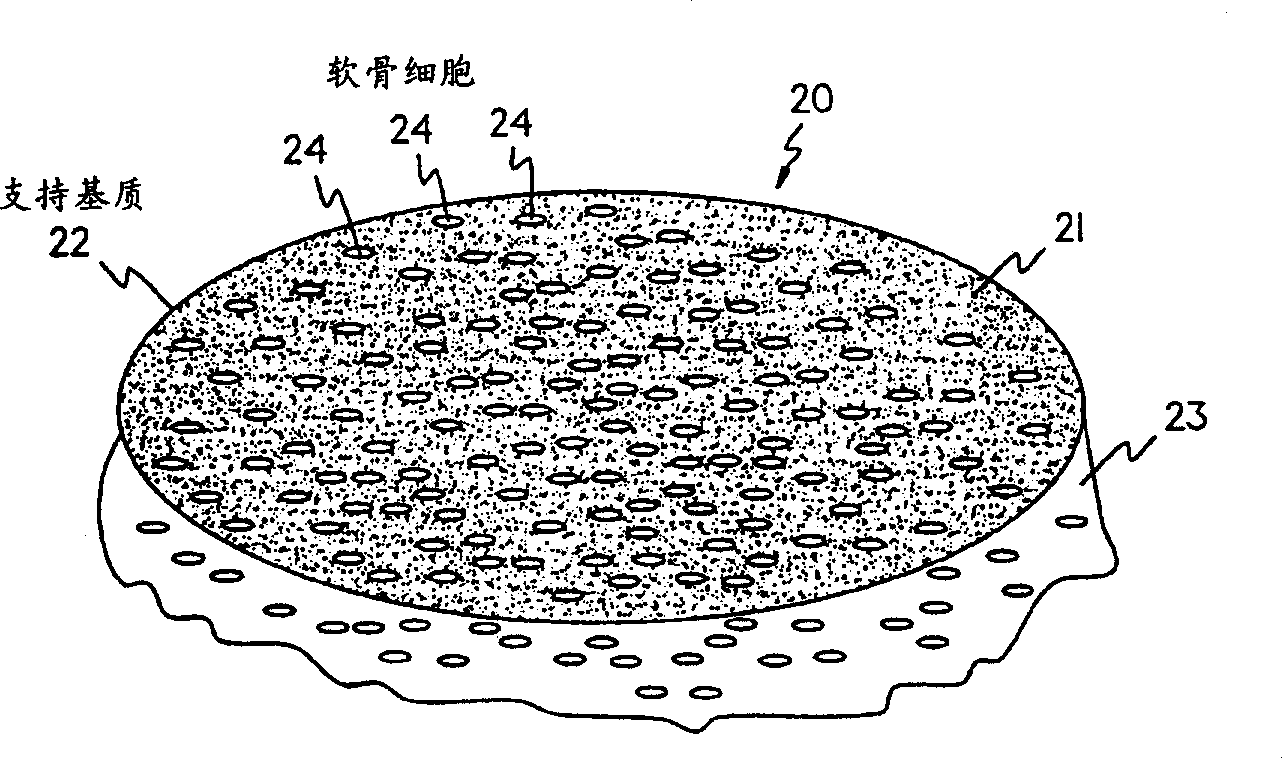
[0069] A support matrix material, specifically Chondro-Gide Collagen membrane cut into with NUNCLON TM An appropriate size that fits the bottom of a well in a cell culture dish. In this example, a circular membrane approximately 4 cm in size was placed at the bottom of the well under aseptic conditions.
[0070] Three weeks later, the chondrocytes were transferred from the growth mediu...
Embodiment 2
[0073] in CO 2 Chondrocytes were cultured in the above growth medium for three weeks at 37°C in an incubator and operated in a class 100 laboratory at Verigen Transplantation Service ApS, Copenhagen, Denmark or at the University of Lübeck, Germany. Cells were trypsinized with EDTA for 5 to 10 minutes and counted in a Bürker-Türk chamber stained with trypan blue viability. Adjust the cell number to 5 x 10 per mL 5 cartilage cells. Place a piece of NUNCLON in the level 100 lab TM Plate open the cover.
[0074] As in Example 1, the Chondro-Gide Support matrix cutting into and NUNCLON TM An appropriate size that fits the bottom of a well in a cell culture dish. In this example, a circular membrane approximately 4 cm in size was placed at the bottom of the well under aseptic conditions.
[0075] Three weeks later, the chondrocytes were transferred from the growth medium to the graft medium described above, and approximately 5 × 10 5 Place the individual chondrocytes direc...
Embodiment 3
[0079] in CO2 Chondrocytes were cultured in the above growth medium for three weeks at 37°C in an incubator and operated in a class 100 laboratory at Verigen Transplantation Service ApS, Copenhagen, Denmark or at the University of Lübeck, Germany. Chondrocytes were trypsinized with EDTA for 5 to 10 minutes and counted in a Bürker-Türk chamber with a trypan blue viability stain. Adjust the cell number to 5 x 10 per mL 5 cartilage cells. Place a piece of NUNCLON in the level 100 lab TM Plate open the cover.
[0080] As in Example 1, the Chondro-Gide Support matrix cutting into and NUNCLON TM An appropriate size that fits the bottom of a well in a cell culture dish. In this example, a circular membrane approximately 4 cm in size was placed at the bottom of the well under aseptic conditions.
[0081] Three weeks later, the chondrocytes were transferred from the growth medium to the graft medium described above, and approximately 5 × 10 5 Place the individual chondrocytes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com