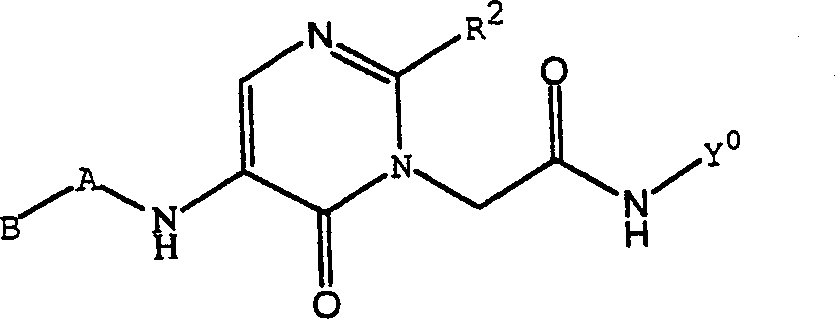
Substituted polycyclic aryl and heteroaryl pyrymidinones useful as anticoagulants agents
A ring carbon and alkyl technology, applied in the direction of blood diseases, antineoplastic drugs, cardiovascular system diseases, etc., can solve problems such as ischemic necrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0580] (EX-1A) 300.0ml of anhydrous methanol solution (1.68M) of ethyl benzimidate hydrochloride (92.25g, 496.9mmol) was cooled to about 0°C, and aminoacetaldehyde was added dropwise at such a rate Dimethyl acetal (73.10 mL, 670.9 mmol) was dissolved in anhydrous methanol (75.0 mL, 9.0 M), keeping the temperature below 5°C. The resulting solution was stirred for 3 days, keeping the temperature below 5°C. The reaction mixture was then concentrated under reduced pressure to give a yellow oil. The residue was dissolved in 1N NaOH (750ml) and extracted with dichloromethane (4 x 250ml). The organic solutions were combined and dried (MgSO 4 ), concentrated to give 108.13 g of crude N-(2,2-dimethoxyethyl)benzamidine as a yellow oil. To a solution of crude N-(2,2-dimethoxyethyl)benzamidine (108.13g, 519.2mmol) in anhydrous methanol (125.0ml, 4.2M) was added methoxysulfide in one portion at room temperature. Dimethyl methylmalonate (94.13 g, 540.5 mmol). The resulting mixture was ...
Embodiment 3
[0599] (EX-3A) to [5-amino-2-phenyl-6-oxo-1,6-dihydro-1-pyrimidinyl] acetate (EX-1F) (613.7mg, 2.037mmol) 25.0 ml of acetic acid and phenylacetaldehyde (0.475 ml, 4.060 mmol) were added to 7.0 ml of tetrahydrofuran and dichloromethane solution (1:1, 0.3 M). The solution was cooled to 0°C in an ice bath, and sodium triacetoxyborohydride (1.9131 g, 9.027 mmol) was added in one portion. After stirring for 5 minutes, the ice bath was removed and the reaction mixture was stirred at room temperature for 2 hours. 1N NaOH (5ml) was added to quench the reaction and the mixture was stirred for 5 minutes. The reaction mixture was diluted with 0.5N NaOH (100ml). The aqueous solution was extracted with ethyl acetate (3 x 25ml). The combined organic solutions were washed with 0.5N NaOH (1 x 25ml) and brine (1 x 25ml). Solution drying (MgSO 4 ), filtered and concentrated under reduced pressure. Purification by MPLC (25% ethyl acetate / hexanes) afforded EX-3A as a yellow oil in 74% yield...
preparation Embodiment 4
[0606] EX-4A) Under stirring, 1.1 N of potassium carbonate was added at one time to the solution of 5-nitro-2,4(1H,3H)pyrimidinedione in dimethyl sulfoxide (0.2M). After about 10 minutes, a solution of 1 equivalent of 2-(trimethylsilyl)ethoxymethyl chloride in dimethylsulfoxide was added dropwise. The reaction mixture was then heated to 40°C and stirred for 18 hours. Typical aqueous workup followed by chromatographic purification afforded pure product EX-4A.
[0607] EX-4B) Under stirring, add 1.1 l equivalent of potassium carbonate. After about 10 minutes, a solution of 1 equivalent of methyl bromoacetate in dimethyl sulfoxide was added dropwise. The reaction mixture was then heated to 40°C and stirred for 18 hours. Typical aqueous work-up followed by chromatographic purification affords pure product EX-4B.
[0608] EX-4C) A methanolic solution of 5-nitro-1-SEM-3-methoxycarbonylmethyl-2,4(1H,3H)pyrimidinedione (EX-4B) was degassed with hydrogen. Then 5% Pd / C was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com