Process for converting methanol or dimethyl ether to olefins
A technology of dimethyl ether and methanol, applied in the field of converting methanol or dimethyl ether into olefins, can solve the problems of reducing the yield of ethylene per pass, reducing the overall efficiency of the method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
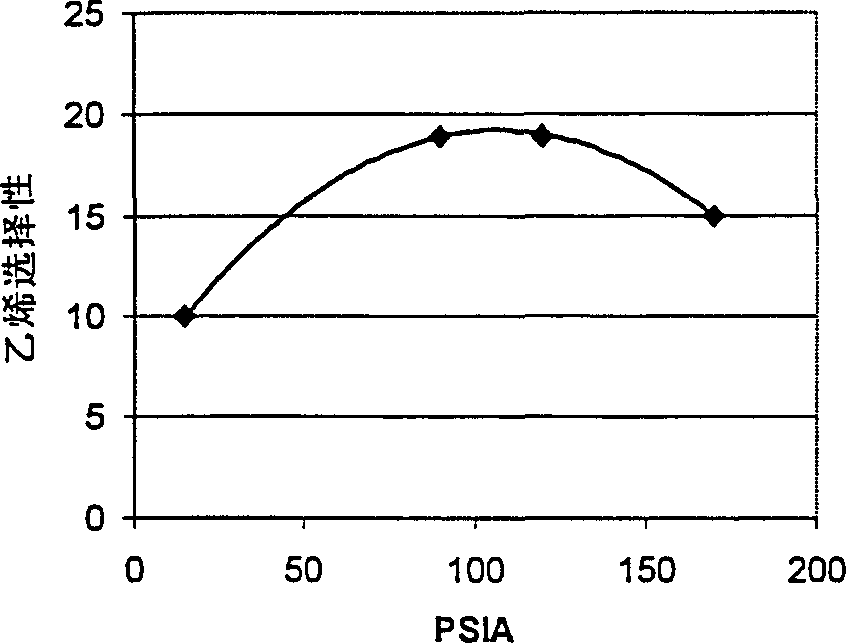
[0047]This example uses an FCC additive catalyst containing ZSM-5 in a silica:alumina mole ratio of 450:1, a binder to zeolite weight ratio of 60:40, and about 4% by weight P . The catalyst had previously been steamed at 870°C (1600°F) for 4 hours so that the catalyst had an alpha value of about 1, a diffusion parameter of 26, and an n-hexane adsorption value of 38 mg / g. Methanol was converted using this catalyst at 430°C and 15, 90, 120 and 170 psia. The ethylene, propylene, C4+ and total olefin selectivities for each condition are listed in Table 1 at about 70% conversion of methanol. figure 1 A plot of ethylene selectivity versus pressure is given in .
[0048] pressure, psia
[0049] Table 1 and figure 1 The results presented in are surprising in that while the overall olefin selectivity is only reduced by 10%, the selectivity to ethylene is increased by more than 60% by increasing the reactor pressure from 15 to 90 psia methanol. This unexpected increase in ...
Embodiment 2
[0050] Embodiment 2 (comparison)
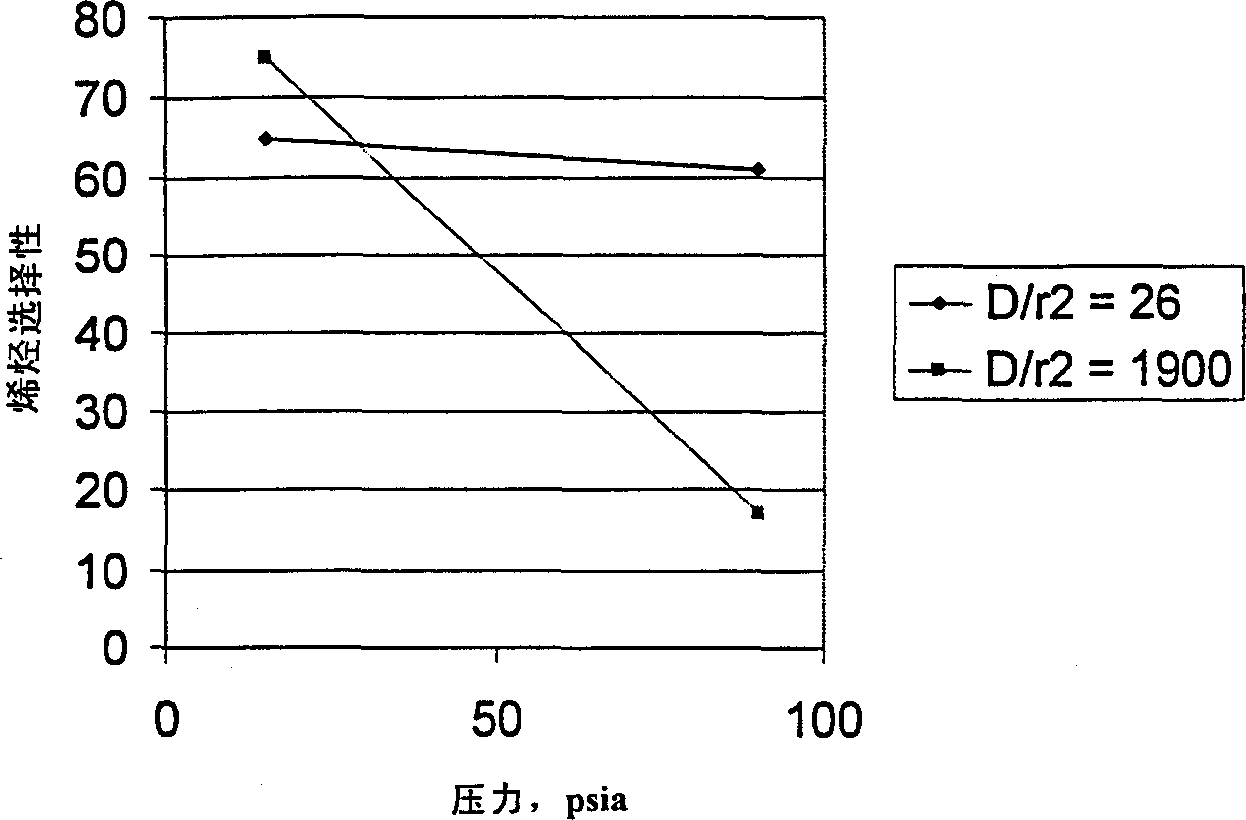
[0051] A ZSM-5 extrudate catalyst containing 65% by weight silica to alumina in a 55:1 molar ratio of ZSM-5 and 35% by weight alumina binder was steamed at 787°C for 45 minutes such that The steamed catalyst had an alpha value of about 4, a diffusion parameter of 1900 and an n-hexane adsorption value of 35 mg / g. Methanol conversion was performed with steamed catalyst at 430°C and methanol partial pressures of 15 and 90 psia. The ethylene, propylene, C4+ and total olefin selectivities for each condition are listed in Table 2 at about 70% conversion of methanol.
[0052] pressure, psia
15
90
temperature, ℃
430
430
ZSM-5 d / r2
1900
1900
methanol conversion
70
70
Selectivity, wt% of HC
Vinyl
2
4
Acrylic
25
7
C4+ olefins
46
6
other HC
27
83
...
Embodiment 3
[0055] Embodiment 3 (comparison)
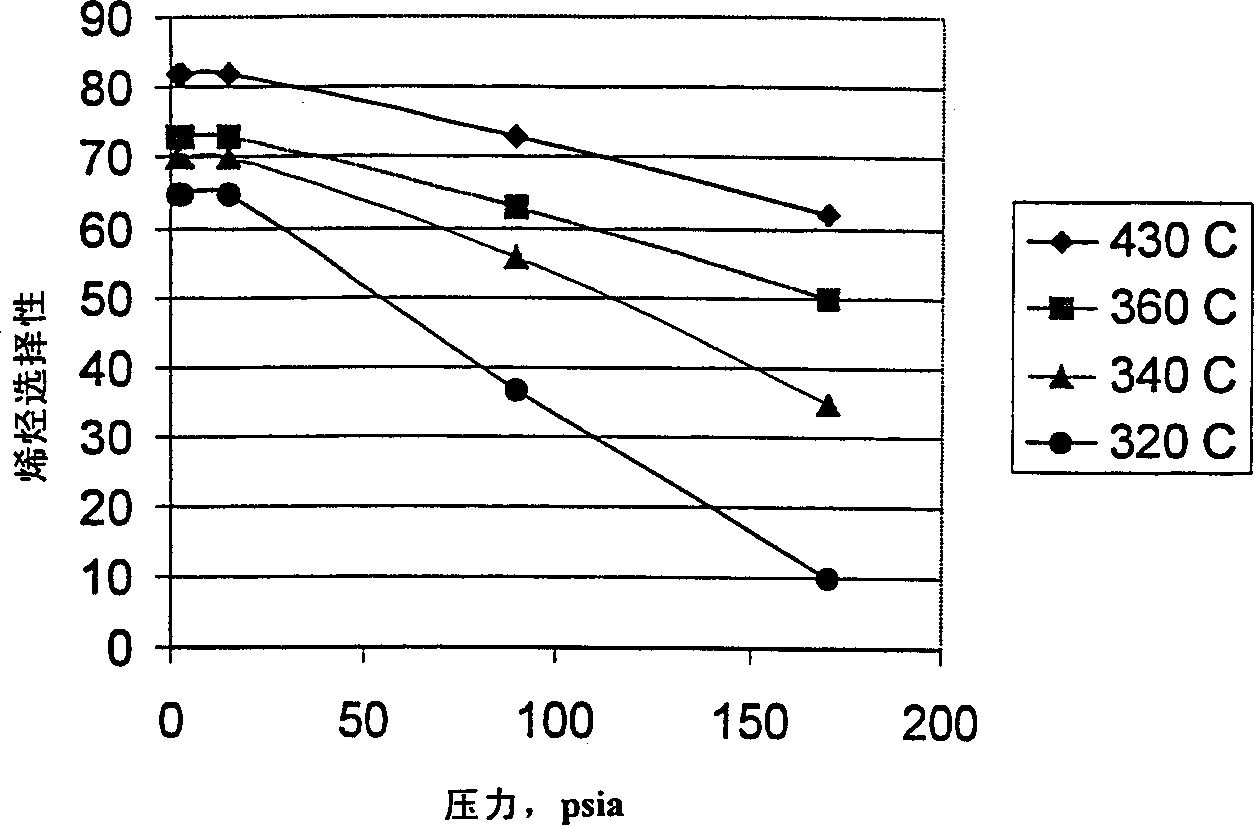
[0056] Phosphoric acid, kaolin, and 450:1 silica / alumina ZSM-5 were slurried in water and spray dried to produce a fluidized bed catalyst. The catalyst was calcined at 510°C in air. The final catalyst contained 40% by weight ZSM-5 and 4.5% by weight phosphorus. The catalyst was then steamed at 1050° C. (1920° F.) for 0.75 hours. After the treatment, the alpha value of the catalyst was about 1, the diffusion parameter was 0.5, and the n-hexane adsorption value was 31 mg / g. The catalyst was used to convert methanol at 480°C, 15 psia and 330°C, 15 psia. The ethylene, propylene, C4+ and total olefin selectivities for each condition are listed in Table 3 at about 70% conversion of methanol.
[0057] pressure, psia
[0058] The data in Table 3 show that even with ZSM-5 catalysts with low diffusion parameters, the ethylene selectivity at low methanol partial pressures decreases significantly with increasing temperature. On the contrary...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com