Process for preparing mesoporous Si-Al catalysis material
A silicon-alumina material and technology for the preparation process, which are applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of complex preparation process, difficult control, expensive raw materials, etc., and achieve simple operation, Easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The specific embodiment embodiment 1 preparation of silicon-alumina catalytic material
[0012] A certain amount of water glass (SiO 2 0.39mol / L, Na 2 O0.125mol / L) solution and 0.96M Al(NO 3 ) 3 The solutions were mixed, ammonia water was added dropwise until the pH of the system was 8, stirred for one hour, filtered, and washed with water and ammonium nitrate solution for 7 times respectively. After filtering, add 200ml water and a certain amount of 0.94M HNO 3 The solution made the pH of the system = 4, and after stirring for 12 hours, a clear silica-alumina sol was obtained. Dry under vacuum conditions (vacuum degree 60 mmHg), and then bake at 550° C. for 8 hours to obtain a silicon-aluminum material.
Embodiment 2
[0014] First, use nitric acid (0.94M) to dissolve 50ml of water glass solution (SiO 2 0.78mol / L, Na 2 (00.25mol / L) pH value is adjusted to about 10, then according to the silicon-aluminum ratio of required preparation material, the aluminum sol freshly prepared is added in the water glass solution after the acidification under the condition of vigorous stirring. After adding a certain amount of nitric acid (0.94M) to dissolve the gel, a light blue silica-alumina mixed sol was prepared. When the sol automatically gels at room temperature, immediately add 70ml NH 4 NO 3 The solution (1.2M) was added to the gel for ion exchange to remove sodium ions from the gel. This process is generally repeated three times. Finally, the obtained gel sample was directly put into a muffle furnace and fired at 550°C for 10 hours to obtain the desired material. The texture data of the synthesized materials are shown in Table 1 below.
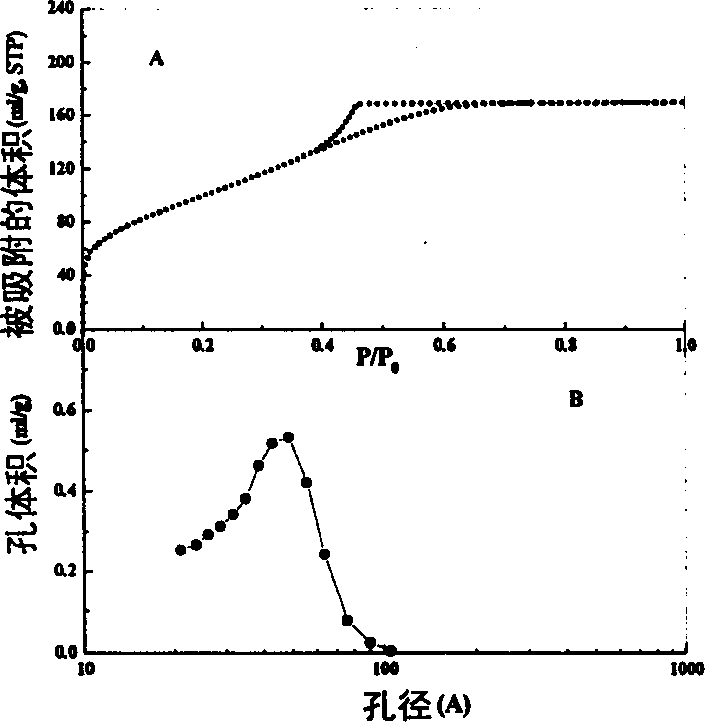
[0015] Table 1 Sample Si / Al BE...
Embodiment 4
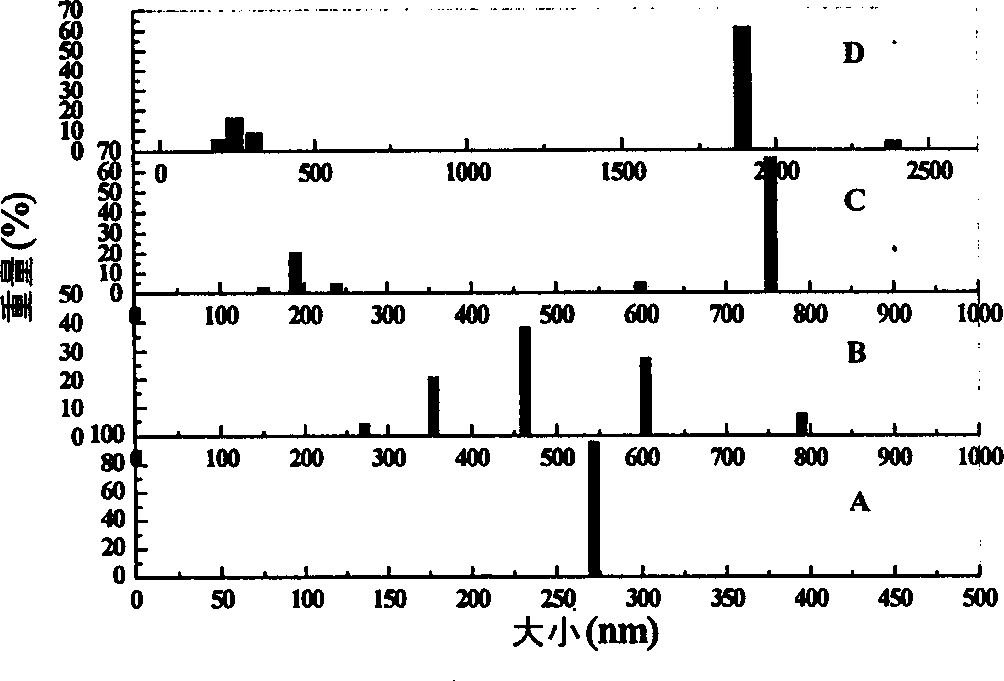
[0016] Coulter N 4 The particle size distribution of the silica-alumina sol prepared in Example 1 was measured by a plus laser particle size analyzer. Figures A, B, C, and D in Figure (1) represent the particle size distribution diagrams of silica-alumina sol samples with different molar ratios of silicon to aluminum (the molar ratios of silicon to aluminum are 1, 4, 7, and 10, respectively). It can be seen from the figure that the particle size of the sol particles in these sol samples is generally larger. And with the increase of the molar ratio of silicon to aluminum, the particles of the sol system gradually increase, and the particle size distribution gradually widens. The above particle size distribution characterization results show that a relatively stable silica-alumina sol can be prepared by this method. Embodiment 4 TEM analysis result
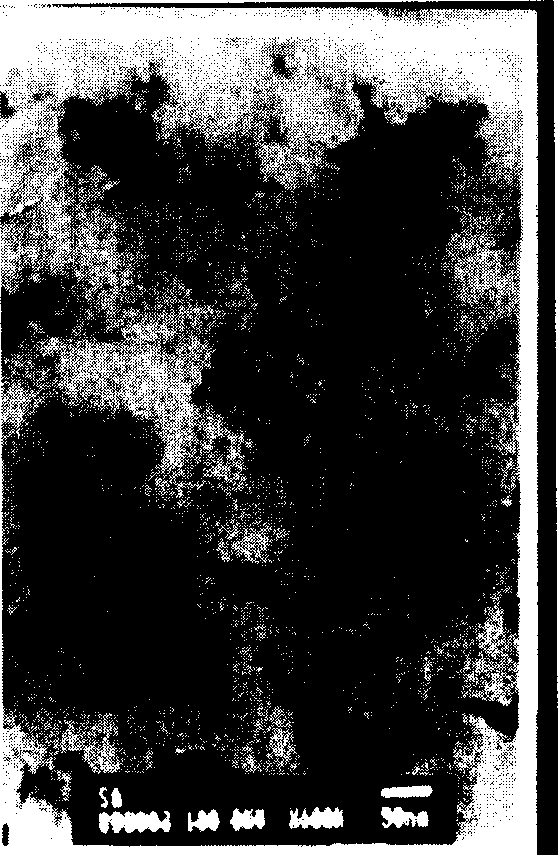
[0017] figure 2 It is the TEM photo of the sol with a silicon-aluminum molar ratio of 1 and the particle size distribution o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com