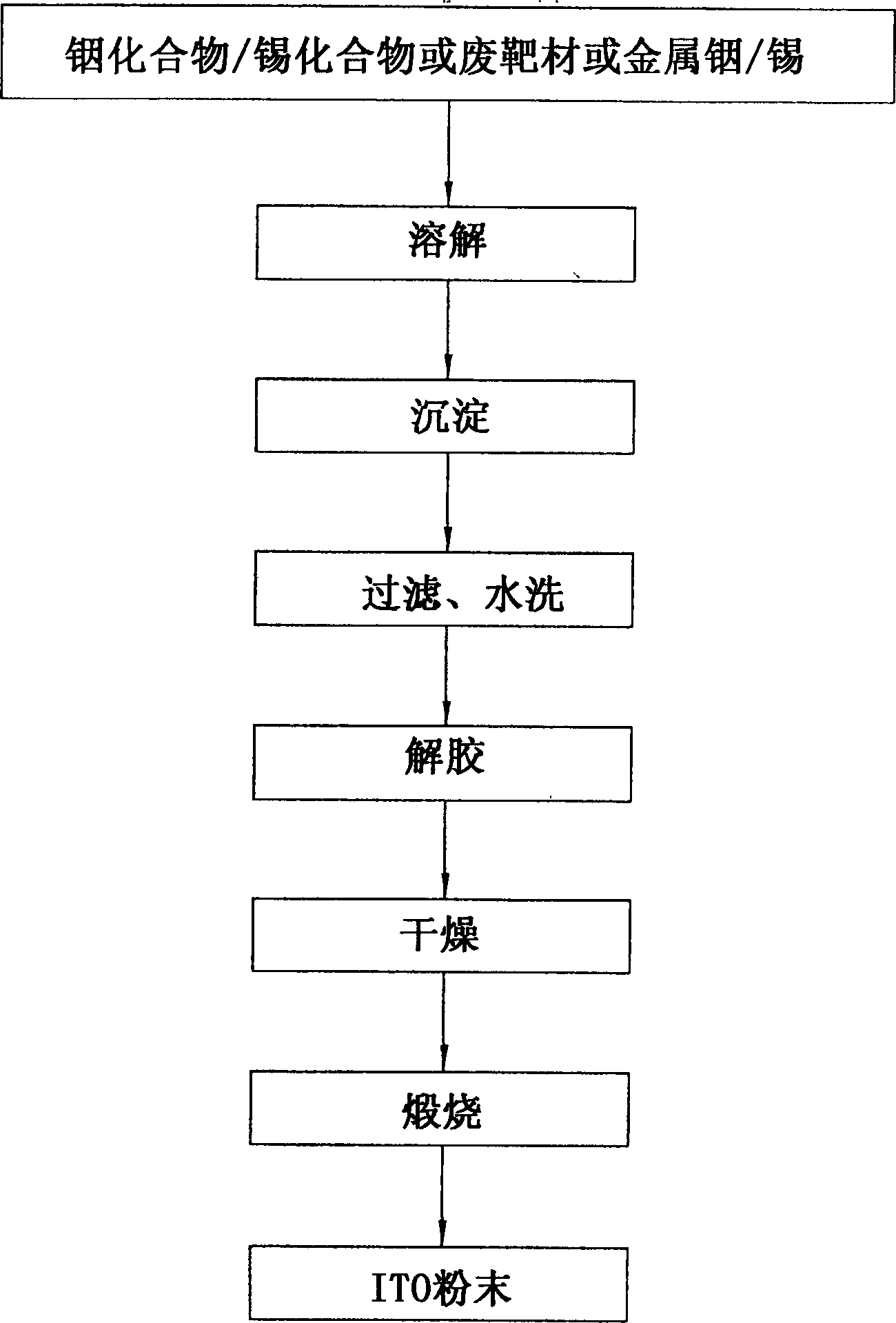
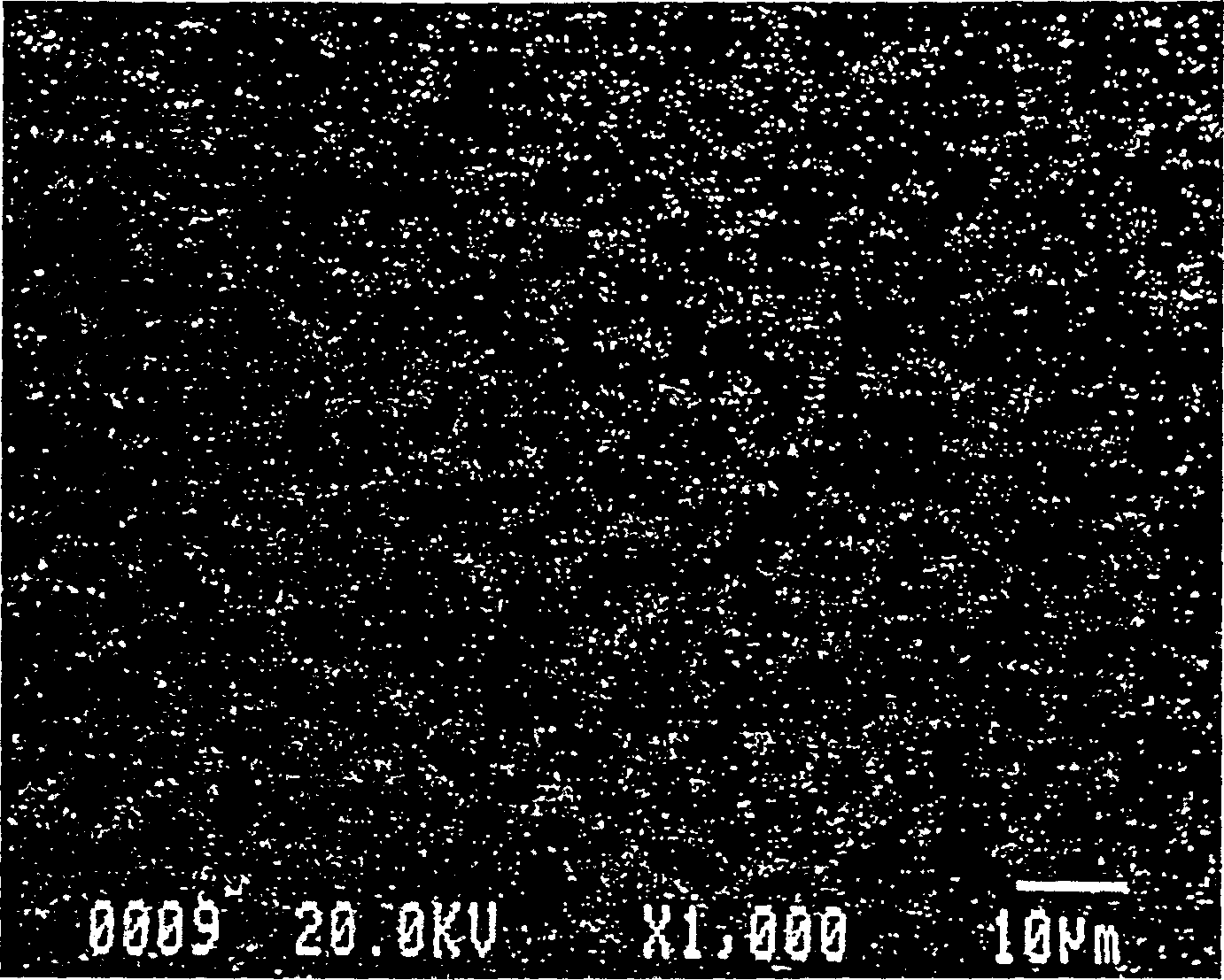
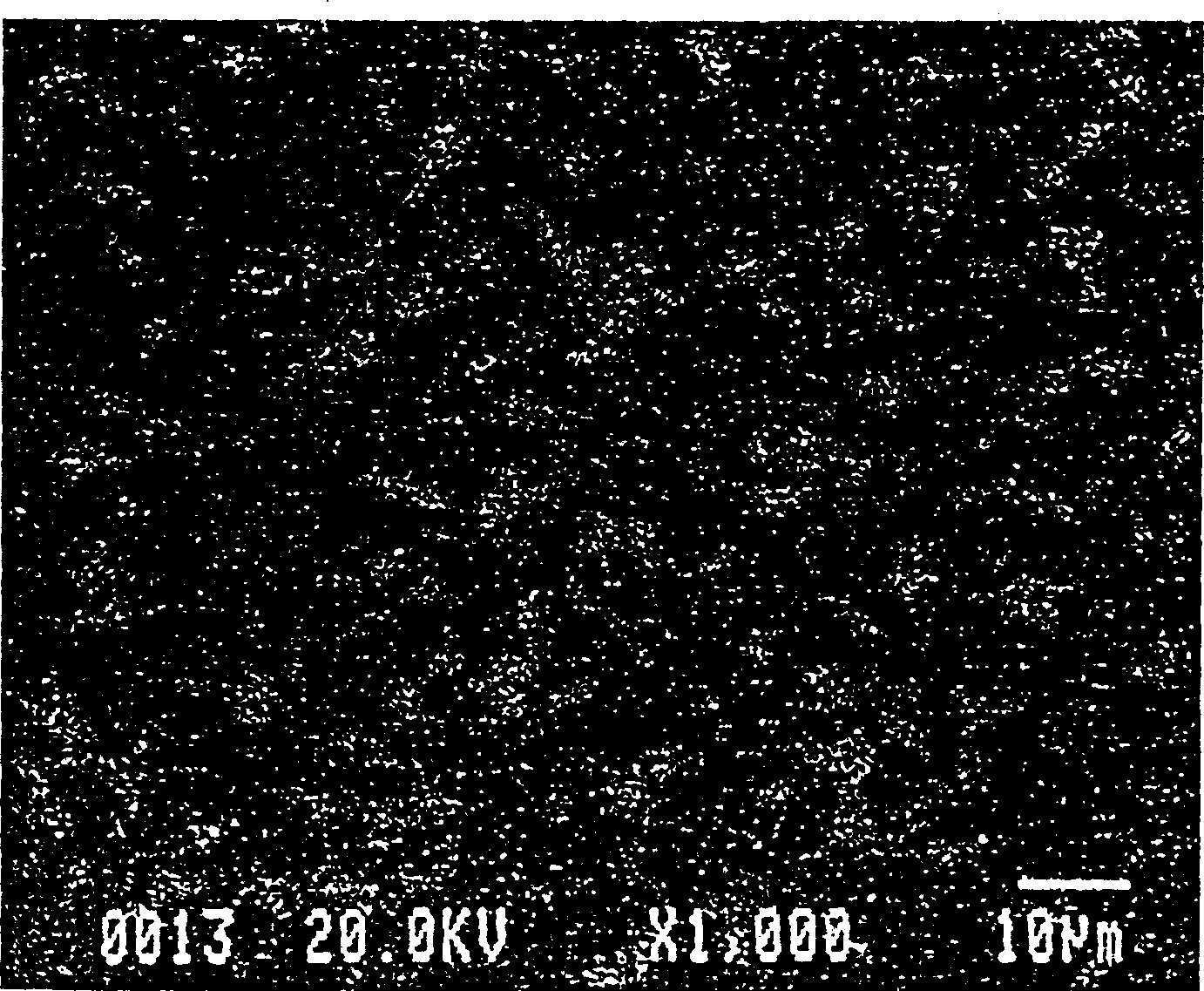
Method for preparing indium tin oxide powder by aqueous solution method
A technology of indium tin oxide and aqueous solution method, which is applied in the fields of tin oxide, chemical instruments and methods, inorganic chemistry, etc., and can solve the problems that the known technology cannot achieve precise preparation, uneven composition, and imprecise preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment one (commercially sold inorganic salt)
[0030] a. Get 50.05g indium nitrate (containing one crystal water, purity 99.99% molecular weight is 318.85) and 5.67g tin chloride (containing five crystal waters, purity 99%, molecular weight is 350.50) and dissolve them in deionized water respectively, and make The final volumes of the solutions were 155 mL and 16 mL respectively, and the concentrations of indium and tin ions were 1.0 M at this time.
[0031] b. Thoroughly mix and stir the above two clear solutions. At this time, the molar ratio of indium tin ions is 95:5.
[0032] c. In the state of stirring, add 35mL concentrated ammonia water (25wt%) quickly, so that the originally clear solution produces white precipitate, and the pH value at this time is 7.25.
[0033] c1. The above solution containing the white precipitate was continuously stirred for at least 12 hours.
[0034] d. Filter the water from the above solution by vacuum filtration to obtain a wh...
Embodiment 2
[0039]Embodiment two (the metal of commercial sale is made inorganic salt)
[0040] a. Take 25.02g metal indium (purity 99.99% molecular weight 114.8), dissolve it in 100mL concentrated nitric acid (70wt%), add appropriate amount of deionized water, make the final volume of the solution be 218mL, and the indium ion concentration at this time is 1.0M. Get 7.94g of tin chloride (containing five crystal waters, purity 99%, molecular weight 350.50) and dissolve it in deionized water, and make the final volume of the solution 22mL, so that the concentration of indium and tin ions are each 1M.
[0041] b. Thoroughly mix and stir the above two clear solutions. At this time, the molar ratio of indium tin ions is 95:5.
[0042] c. In the state of stirring, add 75mL concentrated ammonia water (25wt%) quickly, so that the originally clear solution produces white precipitate, and the pH value is 7.11 at this time.
[0043] c1. The above solution containing the white precipitate was cont...
Embodiment 3
[0049] Embodiment three (metal dissolves into inorganic salt with acid) (comparative known implementation)
[0050] a. Take 25.11g metal indium (purity 99.99%, molecular weight is 114.8), dissolve in 100mL concentrated nitric acid (70wt%), add appropriate amount of deionized water, make the final volume of the solution be 218mL, and the indium ion concentration is 1.0M at this moment. Get 7.96g tin chloride (containing five crystal waters, purity 99%, molecular weight is 350.50) and dissolve in deionized water, and make the final volume of solution be 22mL, make indium, tin ion concentration each be 1M.
[0051] b. Thoroughly mix and stir the above two clear solutions. At this time, the molar ratio of indium tin ions is 95:5.
[0052] c. In the state of stirring, add 100mL concentrated ammonia water (25wt%) quickly, so that the originally clear solution produces white precipitate, and the pH value is now 7.16.
[0053] c1. The above solution containing the white precipitate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com