Process for recovery of organic acids from aqueous solutions
An organic acid, aqueous solution technology, applied in the direction of organic chemistry, solution crystallization, chemical recovery, etc., can solve problems such as increasing the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
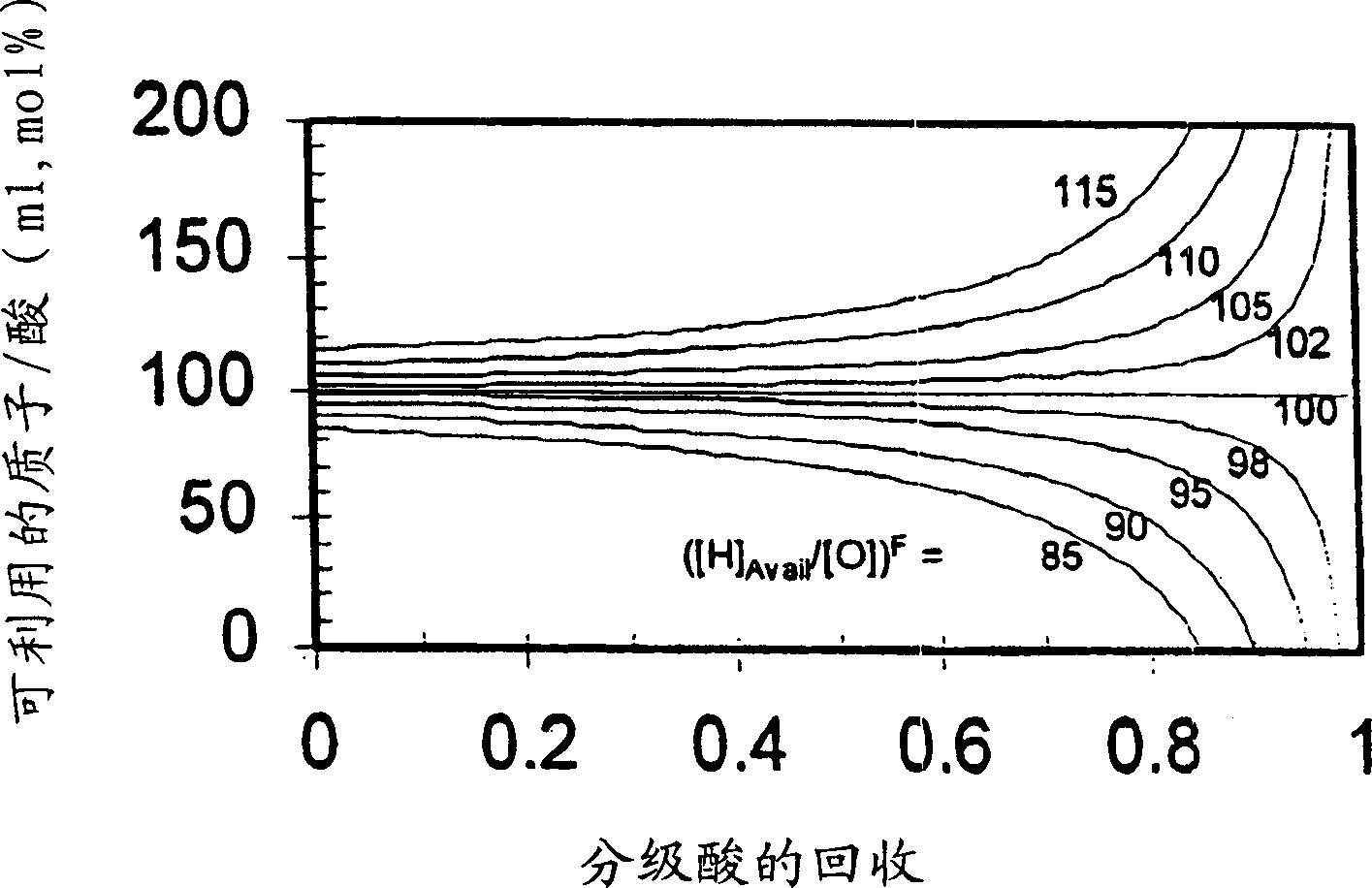
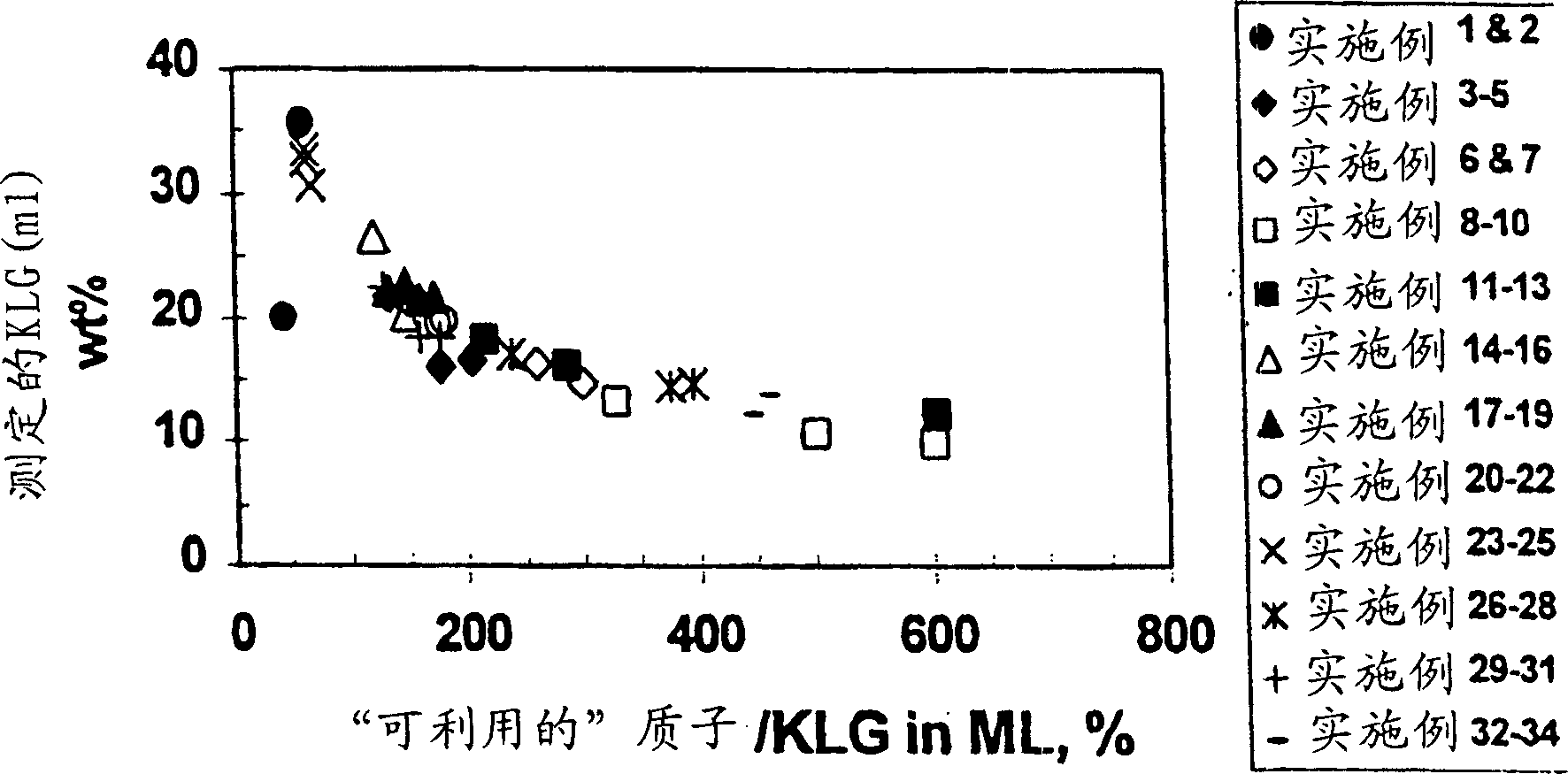
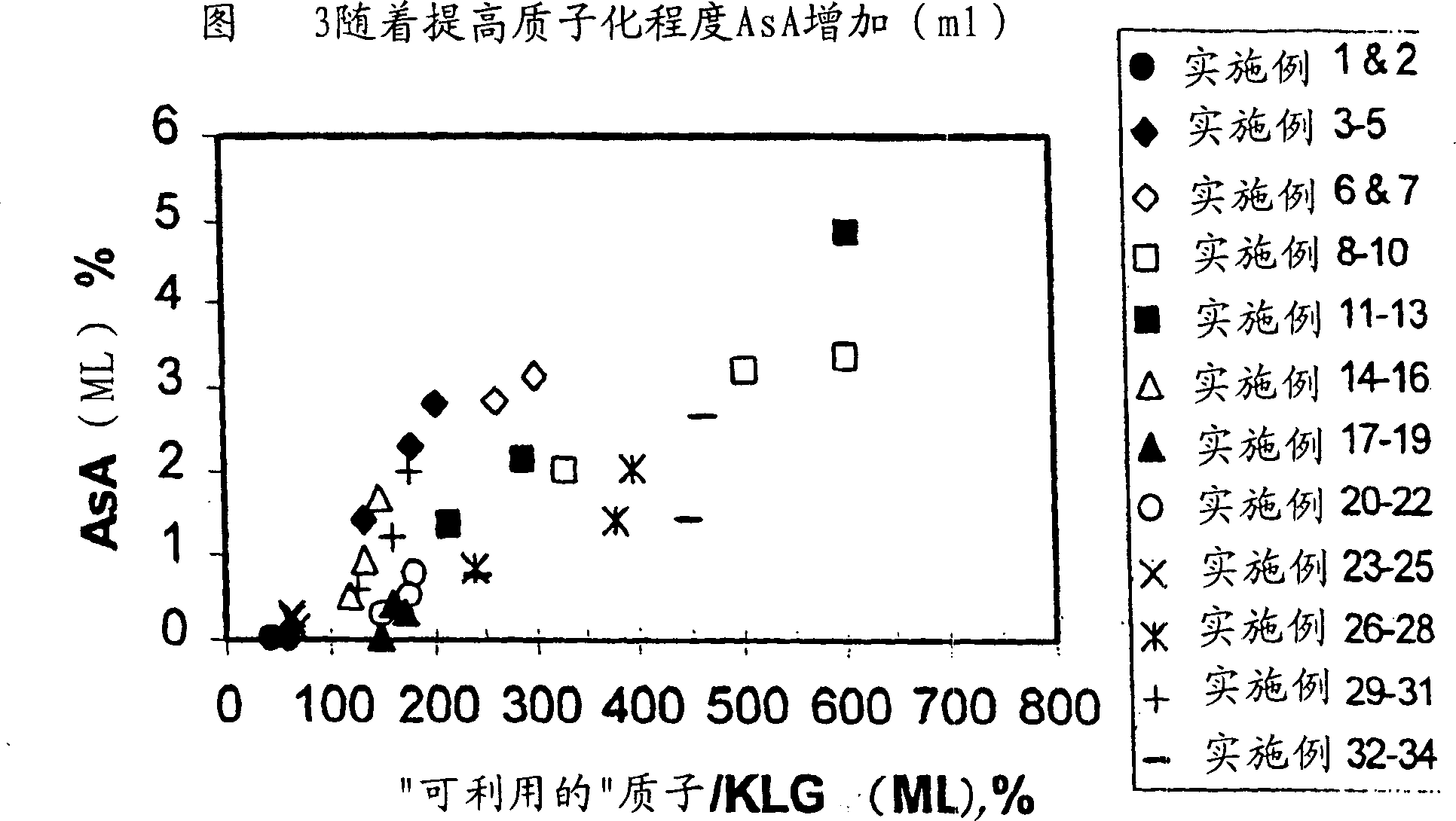
[0105] After acidifying to pH about 2 with sulfuric acid, activated charcoal treatment and microfiltration to remove cells, carbon and other solids, 3031 grams of broth medium and the components listed in Table 1 were evaporated and crystallized at 10°C, and the broth was evaporated to The average concentration of soluble inorganics (K, Na and P) in the mother liquor or filtrate increased by a factor of 7.52 (relative to that in the starting material), and 2312.6 grams of distillate were recovered, vacuum filtered, washed with methanol, and after drying, 361.1 grams were recovered 86.6 wt% pure (anhydrous) KLG product, equivalent to recovery of 77.9% KLG. The components in the mother liquor are listed in Table 2. A comparison of the ratio of available protons to KLG was determined by charge balance in the stock (87.5%) versus in the mother liquor (60.9%), revealing that the protonation of the stock broth was not Sufficiently, even more protons are missing when KLG is crystalli...
Embodiment 2
[0107] Imitating the circulating mother liquor in the continuous crystallization process, 160 grams of the mother liquor from broth crystallization described in Example 1 was combined with 2441.2 grams of the same fresh raw material, and evaporated and crystallized at 10° C. The broth was evaporated until the average concentration of soluble inorganics (K, Na and P) in the mother liquor or filtrate increased by a factor of 6.00 (relative to that in the fresh material), 1772.2 g of distillate was recovered, vacuum filtered, washed with methanol, After drying, 185.23 grams of 85.9 wt% pure (anhydrous) KLG product was recovered, corresponding to a recovery of 41.8% KLG. The components in the mother liquor are listed in Table 2, revealing that the mother liquor of Example 1 was less protonated than the fresh feedstock (60.9% vs. 87.5%) and that the ratio of available protons to KLG was even lower than in the mother liquor. Low (43.5%) is not surprising, this insufficient protonati...
Embodiment 3
[0109] Acidification to pH about 2 using sulfuric acid, charcoal treatment and microfiltration to remove cells, carbon and other solids, contact with H-form cation exchange resin for further pretreatment and the same broth as fresh material in Examples 1 and 2, The broth components obtained are listed in Table 1, wherein 3000.5 grams carry out evaporative crystallization at 10 ℃, and the broth is evaporated to a factor of 4.90 (relative to that in the raw material) that the concentration of phosphorus in the mother liquor or filtrate increases, and 2240 grams of distillation are recovered. liquid, vacuum filtered, washed with methanol, and after drying, 215.0 g of 88.1 wt% pure (anhydrous) KLG product was recovered, corresponding to a recovery of 52.6% KLG. The composition of the mother liquor is listed in Table 2. Unlike Example 1, the ratio of available protons to KLG in the feedstock (112.1%) compared to that in the mother liquor (132.4%) reveals an excess of stock broth. P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com