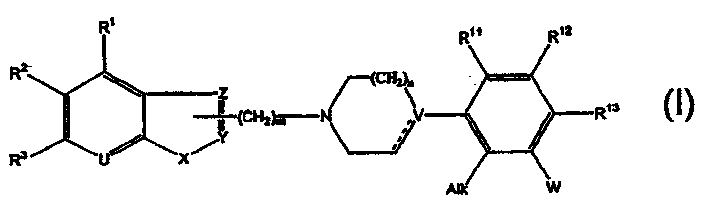
Phenylpiperazinyl derivatives
A technology of phenylpiperazine and derivatives, applied in the field of novel substituted phenylpiperazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] Preparation of intermediates
[0108] A. Preparation of Arylpiperazines
[0109] Hexafluorophosphate η 6 -1,3-dichlorotoluene-η 5 -Cyclopentadienyliron(II)
[0110] Ferrocene (167 g), anhydrous aluminum trichloride (238 g) and aluminum powder (24 g) were suspended in 1,3-dichlorotoluene (500 mL) and heated to 110° C. for 5 hours under nitrogen atmosphere. The mixture was cooled to room temperature and water (1000 mL) was added very carefully in small portions while cooling on an ice bath. Heptane (500 mL) and diethyl ether (500 mL) were added, and the mixture was stirred at room temperature for 30 minutes. The mixture was extracted with diethyl ether (3 x 300 mL). Aqueous ammonium hexafluorophosphate (60 g in 50 mL of water) was added in small portions to the remaining aqueous phase and the product was allowed to precipitate overnight at room temperature. The precipitate was filtered off and dried to give 150 g (39%) of the product as a light green powder. ...
Embodiment 1
[0139] 1-(2-Methyl-3-[3-(dimethylamino)phenoxy]phenyl)-4-[3-(1H-indol-3-yl)-propane Base] piperazine (1a)
[0140] Mix 3-(3-bromopropyl)-1H-indole (2 g), potassium carbonate (1.8 g) and 1-(3-[3-(dimethylamino)phenoxy]- 2-Methylphenyl)piperazine (2g) and boiled at reflux for 5 hours. After cooling to room temperature, silica gel (7 g) was added and the volatile solvent was evaporated in vacuo. The compound was purified by flash chromatography on silica gel (eluent ethyl acetate:heptane:triethylamine (49:49:2)). Fractions containing the compound were combined and concentrated in vacuo. Recrystallization from acetonitrile gave 2 g (66%) of the title compound as white crystals.
[0141] Mp104-105°C. 1 H-NMR (DMSO-d 6 ): 1.80-1.70(m, 2H); 2.15(s, 3H); 240(t, 2H); 2.70(t, 2H); 2.90-2.80(m, 8H); 3.35(s, 6H); 6.03( d, 1H); 6.30(s, 1H); 6.43(m, 1H); 6.55(d, 1H); 6.85(d, 1H); 7.10-6.95(m, 5H); 7.35(d, 1H); 7.50 (d, 1H); 10.75 (br.s, 1H). Analysis (C 30 h 36 N 4 O), calculate...
Embodiment 2
[0153] Example 2 1-{3-[3-(Diethylamino)phenoxy]-2-methylphenyl}-4-[3-(5-fluoro-1H-indol-3-yl)propane Base]piperazine (2a)
[0154] Suspend 2-(3-chlorobutyl)-1,3-dioxolan-4-ylmethoxymethylpolystyrene (70 g, 90.3 mmol) in anhydrous N,N-dimethylformamide (700mL). Sodium iodide (68 g, 452 mmol) was added followed by diisopropylethylamine (232 mL, 1.36 mol) and piperazine (117 g, 1.36 mol). The reaction mixture was heated with stirring at 80 °C for 12 hours. After cooling to room temperature, the resin was filtered and washed with N,N-dimethylformamide (3×500 mL), methanol (3×500 mL), tetrahydrofuran (3×500 mL), followed by methanol and tetrahydrofuran (250 mL each, 5 times) washing. Finally, the resin was washed with dichloromethane (3 x 500 mL) and dried under vacuum (25°C, 36 hours) to give an almost colorless resin (76 g).
[0155] A portion (12.8 g, 16.6 mmol) of the resulting resin was suspended in a 5:1 mixture (150 mL) of anhydrous tetrahydrofuran / N,N-dimethylformam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com