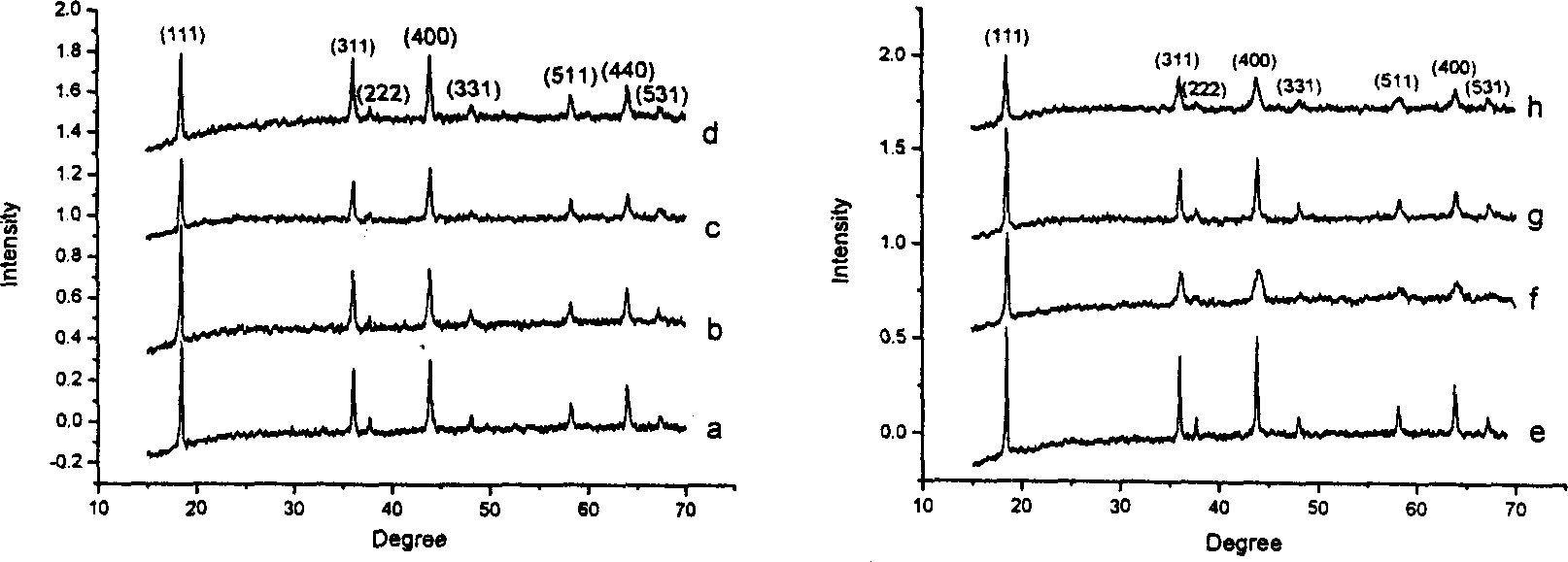
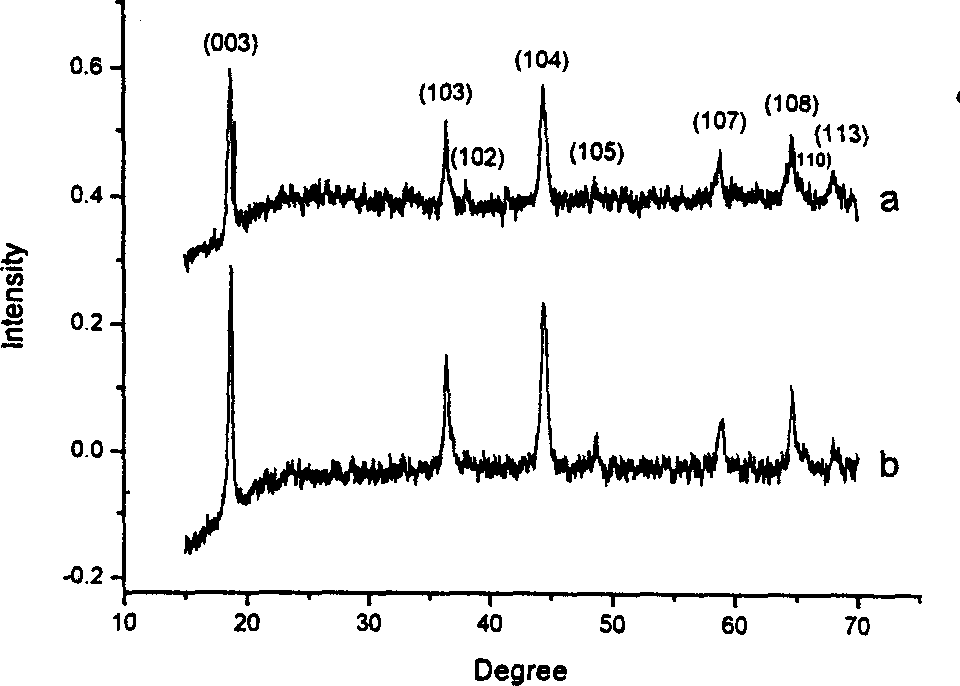
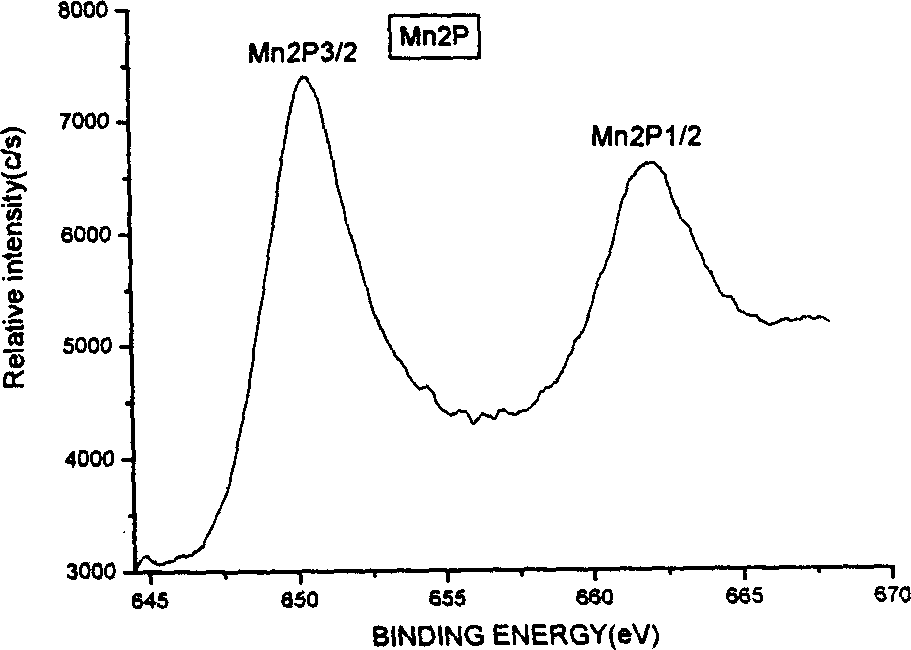
Method for preparing lithium manganese oxide by using low-heat solid phase reaction
A technology of lithium manganese oxide and low-heat solid phase, applied in lithium oxide;/hydroxide, manganese oxide/manganese hydroxide, electrode manufacturing, etc., can solve the problem of difficult control of stoichiometric ratio, high reaction temperature, and product particles Large and other problems, to achieve the effect of high product purity, low synthesis temperature, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. Take by weighing 0.5 gram lithium acetate, 2.2 gram manganese acetate and 2.1 gram oxalic acid respectively, grind about one hour in agate mortar, make three kinds of materials fully mix. The mixture was thoroughly dried at 80°C, and the intermediate product was thoroughly pulverized. Finally, it was placed in a crucible and calcined at 850°C for about 12 hours to obtain black lithium-rich spinel Li 1+x mn 2 o 4 .
Embodiment 2
[0031] Example 2. Weigh 0.5 g of lithium acetate, 2.2 g of manganese acetate, and 0.7 g of oxalic acid, and grind them in an agate mortar for about one hour to fully mix the three substances evenly. The mixture was thoroughly dried at 75°C, and the intermediate product was thoroughly pulverized. Finally, it is placed in a crucible and calcined at 600°C for about 12 hours to obtain black lithium-rich spinel Li 1+x mn 2 o 4 .
Embodiment 3
[0032] Embodiment 3. Take by weighing 0.5 gram of lithium acetate, 2.2 gram of manganese acetate and 5.7 gram of sucrose respectively, grind about one hour in agate mortar, make three kinds of materials fully mix. The mixture was thoroughly dried at 80°C, and the intermediate product was thoroughly pulverized. Finally, it is placed in a crucible and calcined at 600°C for about 15 hours to obtain black lithium-rich spinel Li 1+x mn 2 o 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com