Composite oxide catalyst and its preparing method and use
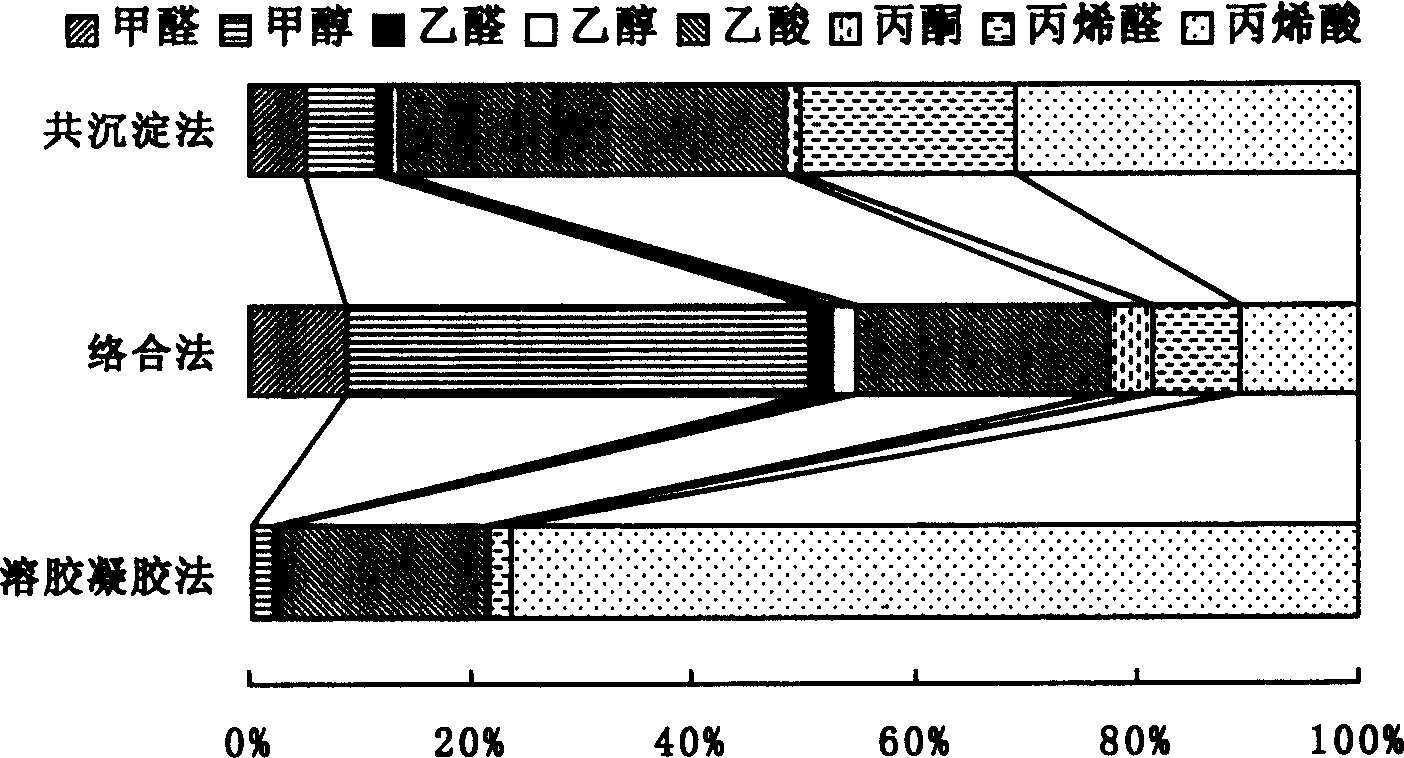
A composite oxide and catalyst technology, applied in catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem that the active components cannot be uniformly dispersed in small grains, the product selectivity cannot be improved, and the The problem of uneven crystal structure of the catalyst can reduce energy consumption, avoid deep oxidation, and prevent excessive oxidation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
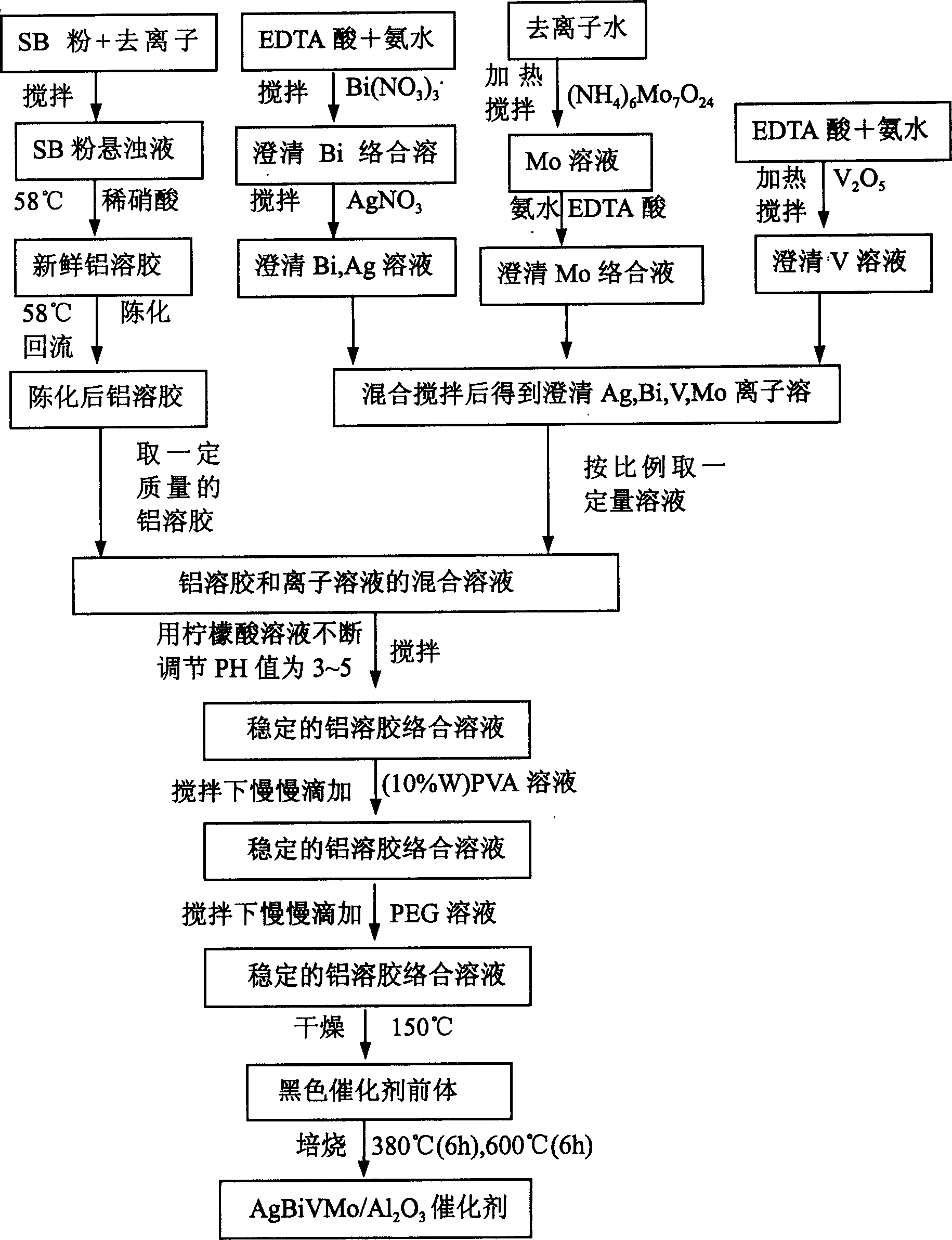
[0052] Embodiment 1-6 (adopting sol mixing technology to prepare Ag-Bi-V-Mo-O / γ-Al 2 o 3 composite oxide catalyst)
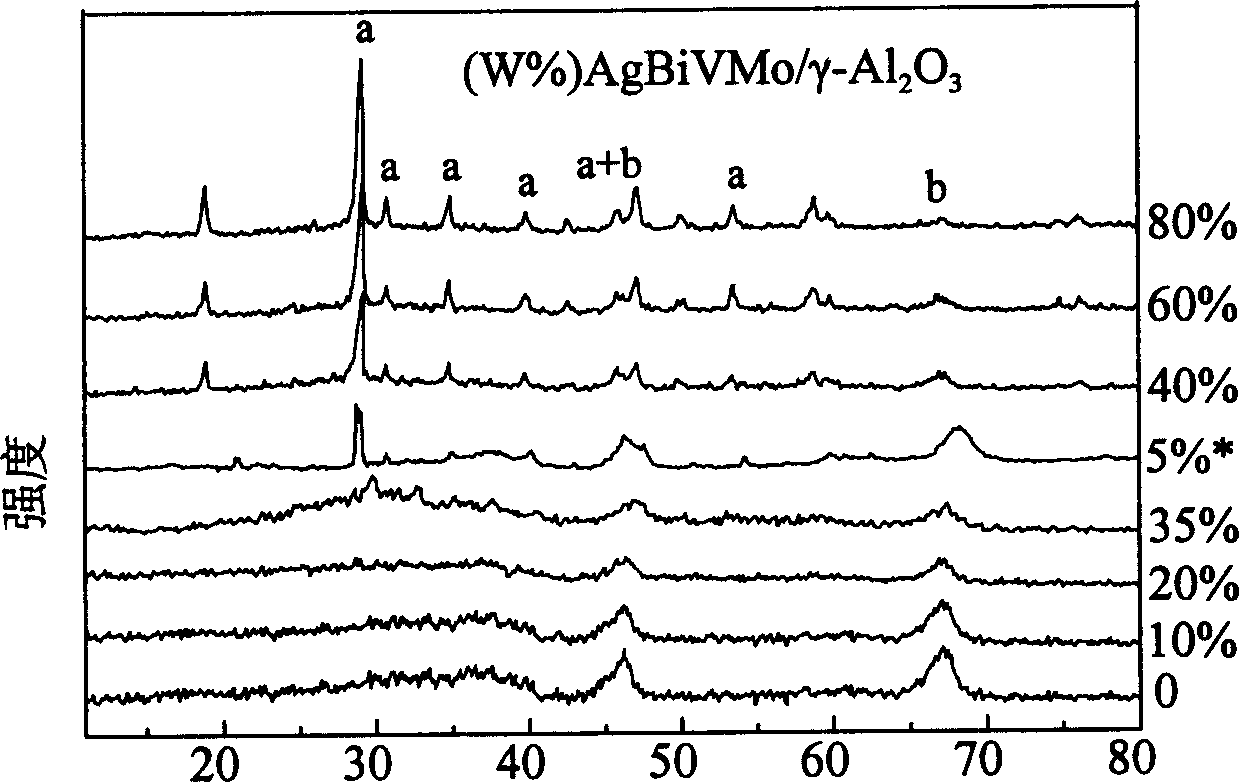
[0053] These examples use the attached figure 1 According to the process flow shown, Ag-Bi-V-Mo-O / γ-Al with active component contents of 10%, 20%, 35%, 40%, 60%, and 80% were prepared 2 o 3 composite oxide catalyst.
[0054] (1) Preparation of stable Ag, Bi, V, Mo ion solutions.
[0055] Add 40.5 grams of EDTA acid (A.R.) to 62.5 milliliters of concentrated ammonia water, stir to obtain a clear and transparent solution, slowly add 41.23 g of Bi(NO 3 ) 3 ·5H 2 O(A.R.) into the solution, stirred until dissolved, then added 0.17 g AgNO 3 Stir to dissolve to obtain solution A.
[0056] Add 20.5 grams of EDTA acid (A.R.) and stir in 70.4 milliliters of concentrated ammonia water to obtain a clear and transparent solution, then add 4.9 grams of V 2 o 5 (A.R.), heated and stirred until completely dissolved to obtain solution B.
[0057] Add 7.94g (NH) 6 Mo...
Embodiment 7
[0064] Embodiment 7 (adopting sol mixing technology to prepare Ag-Bi-V-Mo-Te-O / γ-Al 2 o 3 composite oxide catalyst)
[0065] Weigh 10.0 g H 6 TeO 6 (A.R.) was dissolved in 30ml of distilled water at 80°C and stirred until dissolved. After cooling, it was prepared into 0.1M H with a 100ml volumetric flask. 6 TeO 6 The solution. Prepare Ag by embodiment 1 method, Bi, V, the mixed solution of Mo ion, then 5ml H 6 TeO 6 Add the solution into the mixed solution of Ag, Bi, V, Mo ions and stir, slowly add 40ml of γ-AlOOH sol and stir for 2 hours, evaporate to dryness at 150°C to obtain a black precursor, then bake at 350°C for 6 hours, and then calcine at 600°C Roast for 6 hours.
Embodiment 8
[0066] Embodiment 8 (adopting sol mixing technology to prepare Ag-Bi-Nb-Mo-O / γ-Al 2 o 3 composite oxide catalyst)
[0067] 10g NbCl 5 (A.R.) was dissolved in water, adjusted with ammonia water (1:1) to make the pH value 3-4, and a white precipitate was obtained, which was washed with distilled water until there was no Cl ion. Then dissolve with hot oxalic acid solution (30%) to obtain Nb ion solution. Get 40.5 grams of EDTA acid (A.R.) and add it to 62.5 milliliters of concentrated ammonia water, add 41.23g of Bi(NO 3 ) 3 ·5H 2 O(A.R.) into the solution, stirred until dissolved, then added 0.17 g AgNO 3 Stir to dissolve. After mixing the above two solutions, add 7.94g (NH 4 ) 6 Mo 7 o 24 4H 2 O (A.R.) and 9.5g EDTA acid (A.R.), adjust the pH value to about 9 with ammonia water, slowly add 40ml γ-AlOOH sol and stir for 2 hours, evaporate to dryness at 150°C to obtain a black precursor. Precursor at 350°C Calcined for 6 hours, after calcined at 600℃ for 6 hours, Ag-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com