Methof for preparing l-valine and d-valine by using chemical resolution process
A technology of D-valine and L-valine, which is applied in the field of preparation of chiral organic compounds, can solve problems such as no report on product optical purity and yield, cumbersome resolution process, insufficient product yield and optical purity, etc. problems, to achieve the effects of low production cost, simple synthesis process, and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
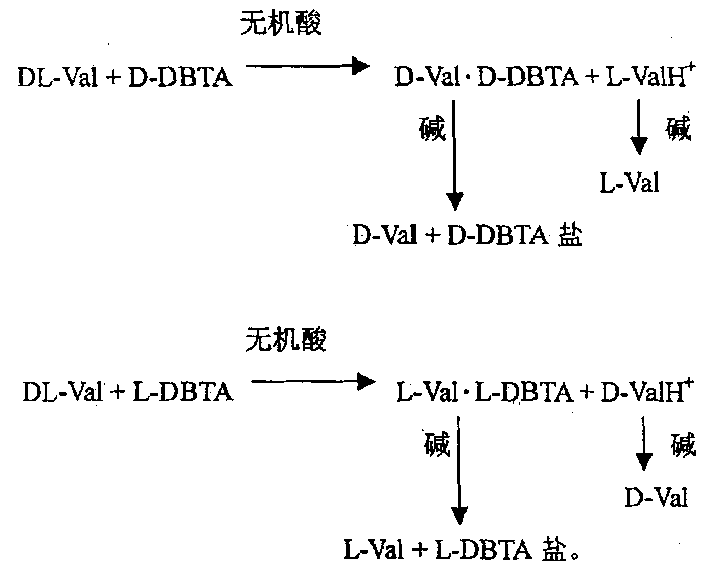
[0033] Dissolve 2.34g of DL-Val in 20ml of dilute hydrochloric acid, heat to 80-100°C under stirring, add 3.58g of L-DBTA, react at this temperature for half an hour, then keep at 60°C for 1 hour, gradually a precipitate forms, and then Cool to about 15°C, filter with suction, dissolve the filter cake in 30ml of ethanol and 2 times the amount of triethylamine, stir for 1 hour, filter with suction, wash with ethanol, dry to obtain 0.94g of L-Val, and the resolution yield is 80.3% , [α] D 22 =+27.5°. Split and concentrate the mother liquor, adjust the pH value to 5.5 with alkali, stir, a white solid precipitates, filter with suction and wash with ethanol, dry to obtain 0.84g of D-Val, the yield is 71.8%, [α] D 22 =-27.5°.
Embodiment 2
[0035] Dissolve 2.34 g of DL-Val in 20ml of dilute hydrochloric acid, stir, heat to 90-100°C, add 3.76g of D-DBTA, react at this temperature for 50 minutes, then keep at 60°C for 1 hour, gradually a precipitate forms, and then cool to At about 15°C, filter with suction and wash with water, dissolve the filter cake in 30ml of ethanol and 2 times the amount of triethylamine, stir for 1 hour, filter with suction, wash with ethanol, dry to obtain 0.94g of D-Val, and the resolution yield is 80.3 %,[α] D 22 =-30°. Split and concentrate the mother liquor, adjust the pH value to 5.5 with alkali, stir, a white solid precipitates, filter with suction and wash with ethanol, dry to obtain 0.83g L-Val, yield 70.9%, [α] D 22 =+27.5°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com