Electrolyte for xecondary battery and secondary battery using said electrolyte
A secondary battery and electrolyte technology, applied in the field of electrolyte, can solve the problems of long-term continuous control of surface film, etc., and achieve the effects of reducing the effect of elution, increasing inhibition, and resistance inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
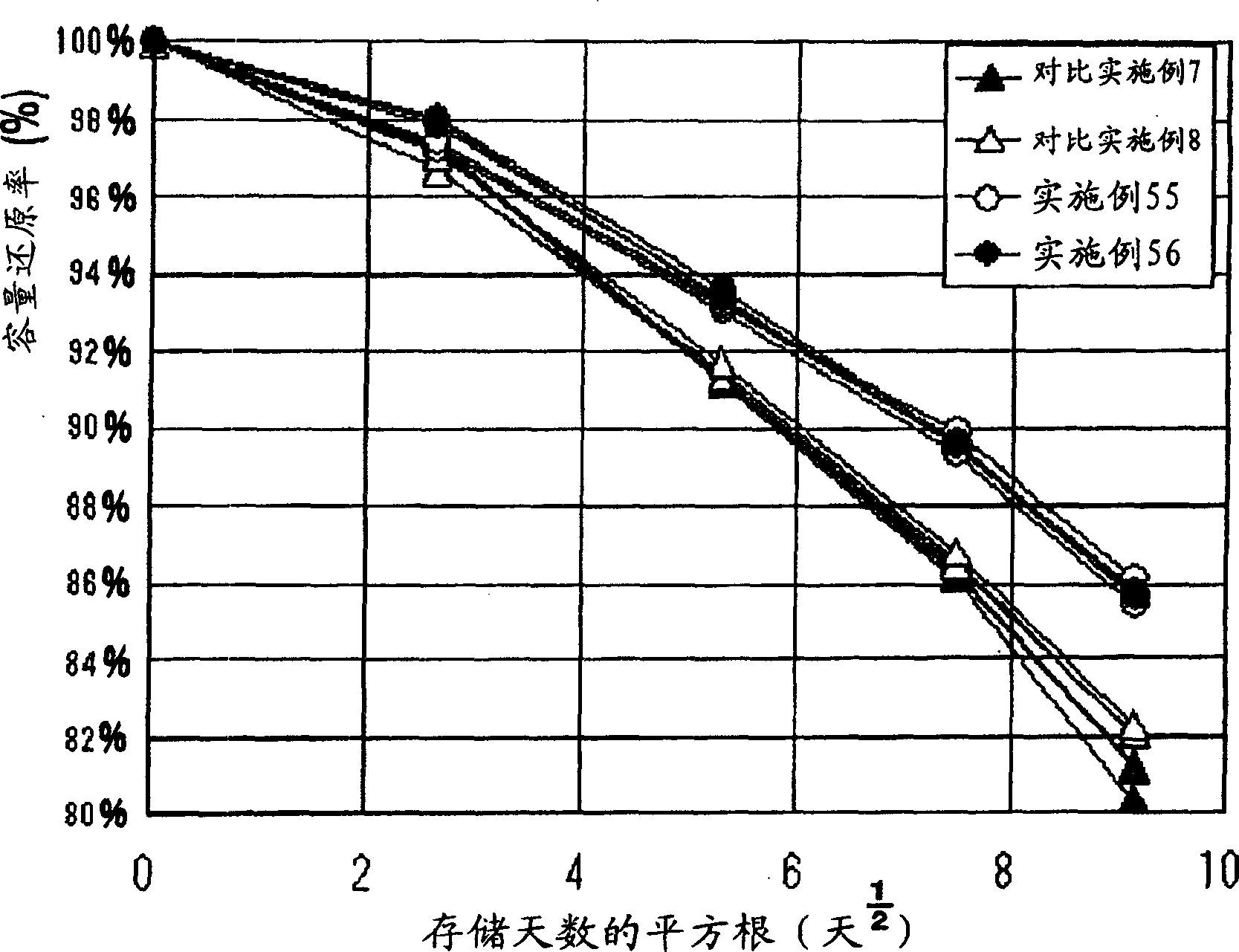
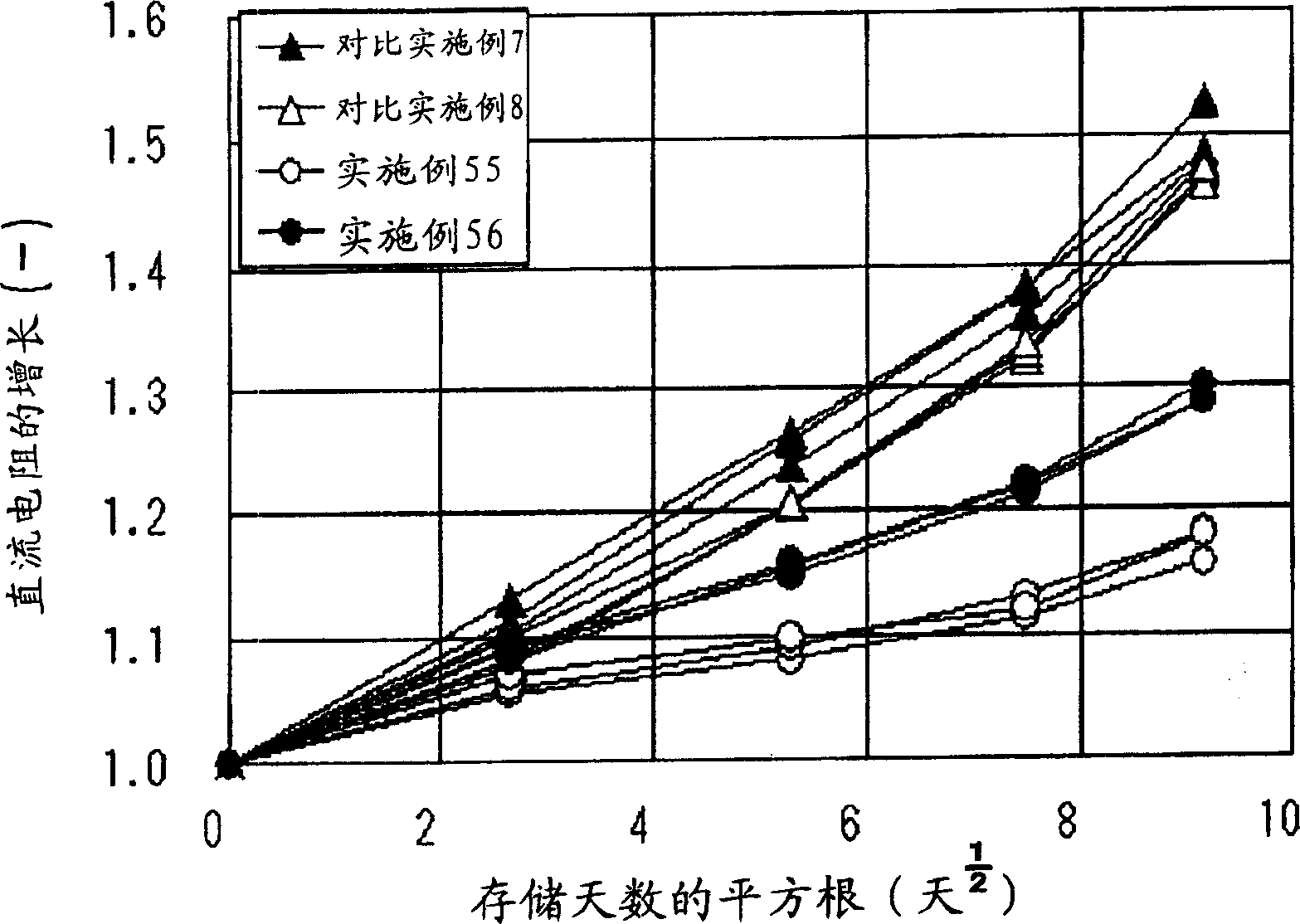
Image
Examples
Embodiment 1
[0098] (Preparation of battery)
[0099] The preparation of the battery according to this example will be described below. Aluminum foil with a thickness of 20 μm was used as the cathode active material, LiMn 2 o 4 Used as an anode active material. Copper foil with a thickness of 10 μm was used as the anode conductive electrode, and metallic lithium was deposited on the copper foil as an anode active material to a thickness of 20 μm to form the anode. Using a mixed solvent of EC and DEC (volume ratio 30:70) as the electrolyte, 1 mole per liter of LiN(C 2 f 5 SO 2 ) 2 (hereinafter abbreviated as LiBETI) was dissolved in the above solvent as a supporting electrolyte, and Compound No. 1 shown in the above Table-1 was also added to obtain a concentration of Compound No. 1 of 1% wt. Then, the anode and the cathode were laminated in which a separator including polyethylene was provided to form a laminate packaged secondary battery.
[0100] (charge-discharge cycle test)
[...
Embodiment 2-4
[0103] A secondary battery was prepared in the same manner as in Example 1 except that Compound No. 1 was replaced with the compounds shown in Table-2. Battery characteristics were also tested in the same manner as in Example 1. The results are shown in Table-2.
Embodiment 5
[0111] A secondary battery was prepared in the same manner as in Example 1, except: LiPF for LiBRTI used to support the electrolyte 6 Alternatively, the anode was prepared as follows: a slurry mixture of graphite powder, a solvent of N-methyl 1-2-pyrrolidone and a binder of a mixture of polyvinylidene fluoride and conductive material dissolved in the solvent were applied on a copper foil, dried produced copper foil. In this example, a cylindrical secondary battery was prepared. Battery characteristics were tested in the same manner as in Example 1. The results are shown in Table-3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com