Novel TNFR-FC fusion protein
A fusion protein and receptor protein technology, applied in the field of genetic engineering, can solve problems such as ineffective prevention and treatment of diseases, difficulty in exerting biological activity, affecting biological activity and stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1. Cloning and expression of novel TNFR-Fc
[0065] The truncated sTNFRII gene sequence was obtained by PCR amplification using the cDNA of human leukocytes as a template, and the enzyme cutting sites at both ends were EcoRI and BamHI.
[0066] Using the cDNA of human splenocytes as a template, the truncated IgG Fc gene sequence was obtained by PCR amplification, and the two ends were BamHI and HindIII.
[0067] The obtained truncated sTNFRII sequence and truncated human IgG Fc sequence were used as templates, and the 5' end primer of the first step and the 3' end primer of the second step were used as primers for PCR amplification to obtain the full-length novel TNFR-Fc gene sequence.
[0068] The PCR product was purified with Qiagen purification column, digested with EcoRI and HindIII, and cloned into the plasmid vector pcDNA3(-).
[0069] Using T7 primers, BGH primers and internal sequencing primers, the nucleotide sequence was determined by an automatic se...
Embodiment 2
[0073] Example 2. Affinity determination of novel TNFR-Fc
[0074] (1) Comparison experiment of receptor ligand binding
[0075] New TNFR-Fc protein and control protein (untruncated TNFR-Fc) were dissolved in TBST buffer, protein A Sepherose beads and 125I-TNFβ were added, and different concentrations of unlabeled TNFβ were added, and shaken at room temperature for 1 hour , centrifuged for 2 minutes to precipitate the combination of beads and TNFR-Fc, and counted with a gamma counter.
[0076] The counting results were analyzed with GraghPad Prism software, and the calculated EC50 was 0.35nM for the new TNFR-Fc protein and 0.54nM for the control protein. The results showed that the two proteins had very close affinity to TNF β. ( figure 2 )
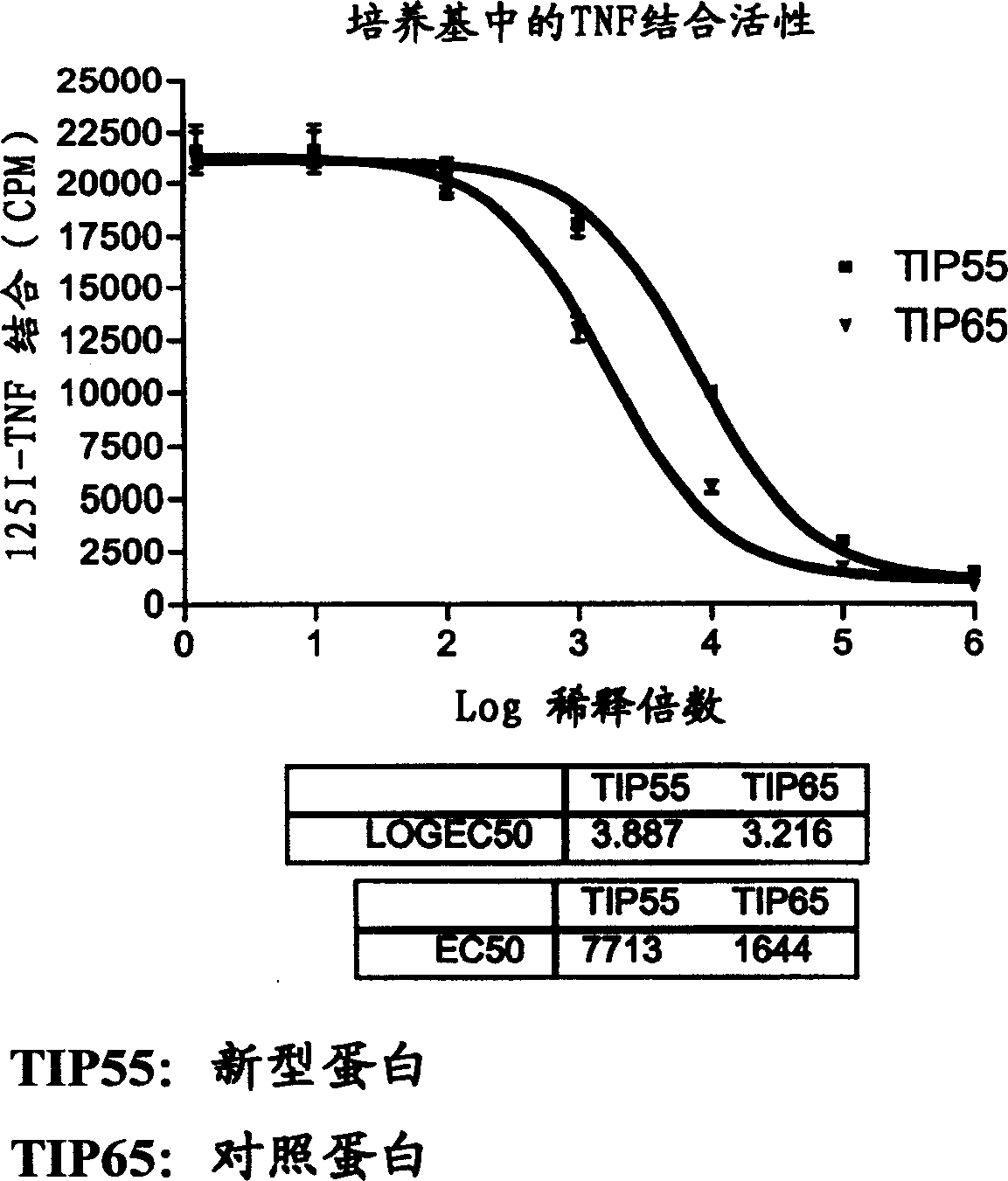
[0077] (2) Comparison experiment of protein concentration in the culture medium of stably expressing cell lines
[0078] The expression cells of novel TNFR-Fc protein and control protein (untruncated TNFR-Fc) were cultured in serum-f...
Embodiment 3
[0084] Example 3. Stability determination of novel TNFR-Fc
[0085] The new TNFR-Fc and control protein in PBS buffer were sterilized and filtered with a 0.22um filter membrane, distributed into 5ml glass bottles with rubber stoppers, and placed in a 37°C incubator. The new TNFR-Fc was subjected to reducing SDS-PAGE protein electrophoresis and cell activity test at 57 days. The control protein adds a test point at 28 days except 57 days
[0086] (1) Comparison of electrophoresis results
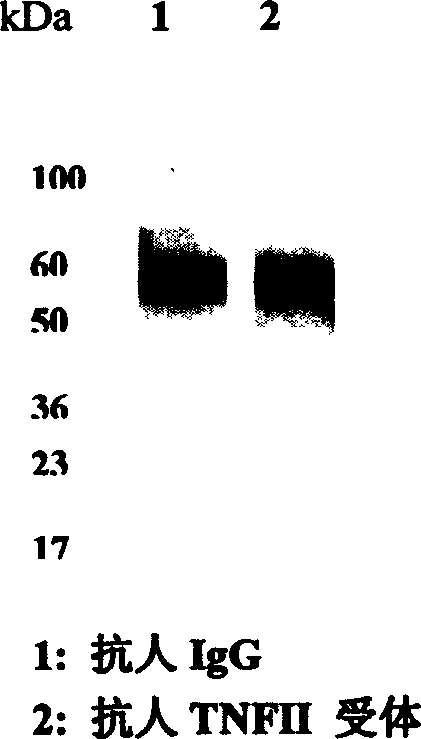
[0087] For protein electrophoresis, load 10ug per well and stain with silver staining. The results showed that the stability of the novel TNFR-Fc was significantly better than that of the control protein. (Figure 5A)
[0088] (2) Comparison of cell activity
[0089] The samples incubated for 57 days were taken for cell viability test, and the results showed that the cell viability of the novel TNFR-Fc protein was 7.59 times that of the control protein after being placed at 37°C for 57 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com