Process for prepn. of dialkyl formanmide
A technology of dialkylformamide and a manufacturing method, which is applied to the preparation of carboxylic acid amides, organic chemical methods, chemical instruments and methods, etc., can solve the problems of difficulty in controlling reaction temperature, accumulation of crystalline by-products, etc. Control becomes difficult, stable continuous production for a long time, and the effect of suppressing accumulation of crystalline by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
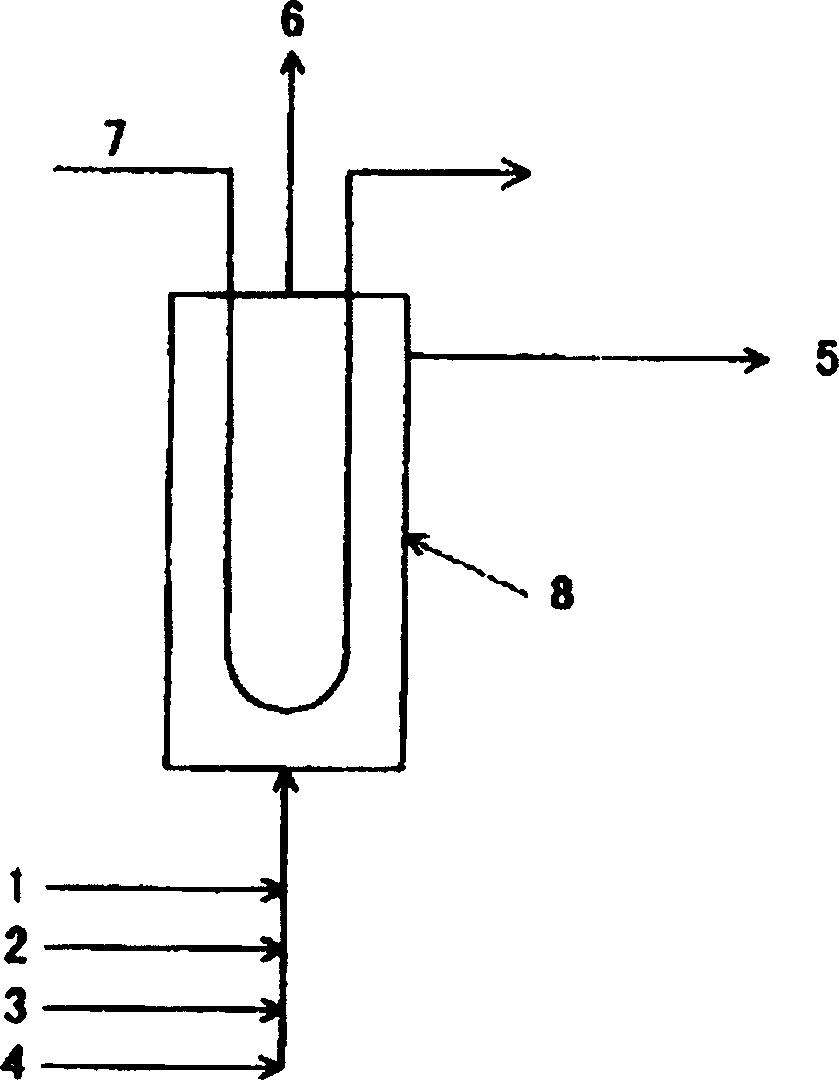
[0029] exist figure 1 In this process, the mixed gas obtained by gasification of coal was purified and adjusted to 40% by volume of carbon monoxide, 58.9% by volume of hydrogen, 1.0% by volume of nitrogen, and 0.1% by volume of oxygen. Supply the mixed gas 2800Nm from the lower part of the reaction tower 3 / hr(Nm 3 / hr is the gas flow rate based on 0°C and 101.3kPa, and the carbon monoxide flow rate based on mass is 1400kg / hr), and 2600kg / hr of dimethylamine containing 200mg of water relative to 1kg of dimethylamine, 260kg / hr of methanol, and 24 56 kg / hr of a methanol solution of sodium methoxide in mass % was reacted at a pressure of 2.0 MPa and a temperature of 115°C. The molar ratio of oxygen / sodium methoxide at this time was 0.5. The reaction product is extracted from the top of the reaction tower as a liquid, and the inert gas is extracted from the top of the tower. The dimethylformamide in the reaction product was 4010 kg / hr, and the conversion rate of dimethylamine ...
Embodiment 2
[0031] exist figure 1 In this process, the mixed gas obtained by gasification of coal was purified and adjusted to 40% by volume of carbon monoxide, 58.7% by volume of hydrogen, 1.0% by volume of nitrogen, and 0.3% by volume of oxygen. Supply the mixed gas 2800Nm from the lower part of the reaction tower 3 / hr(Nm 3 / hr is the gas flow rate based on 0°C and 101.3kPa, and the carbon monoxide flow rate based on mass is 1400kg / hr), and 2600kg / hr of dimethylamine containing 200mg of water relative to 1kg of dimethylamine, 260kg / hr of methanol, and 24 The mass % methanol solution of sodium methoxide was 84 kg / hr, and the reaction was carried out at a pressure of 2.0 MPa and a temperature of 115°C. The molar ratio of oxygen / sodium methoxide at this time was 1.0. The reaction product is extracted from the top of the reaction tower as a liquid, and the inert gas is extracted from the top of the tower. The dimethylformamide in the reaction product was 4010 kg / hr, and the conversion ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com