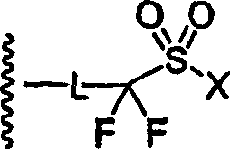
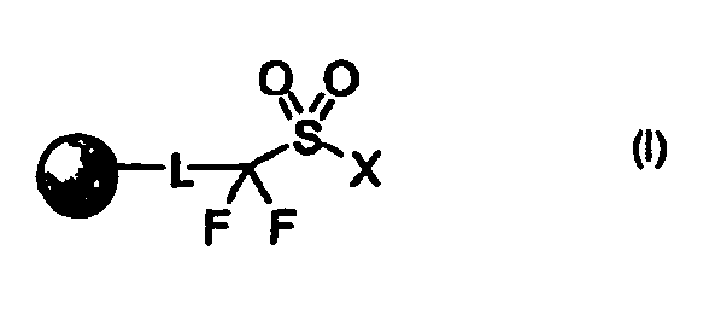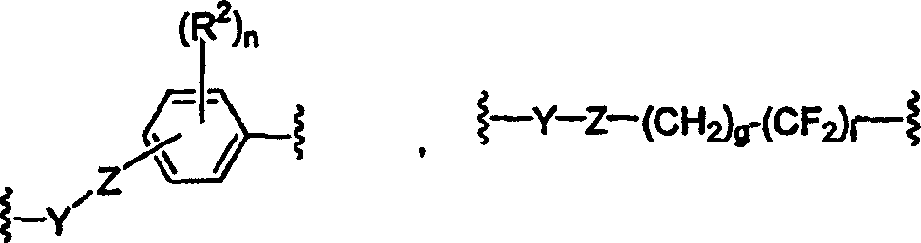Perfluoro sulfonyl halides and related species as polymer support modifiers
一种载体、活化剂的技术,应用在有机化合物/氢化物/配位配合物催化剂、硅有机化合物、化学/物理/物理化学过程等方向,能够解决聚合物不能溶胀等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0398] This example illustrates the preparation of a polymer supported perfluorosulfonyl fluoride linker (Figure 1).
[0399] at 0°C, to the stirred CH 3 CN (8 mL) and H 2 O (7 mL) containing ethyl vinyl ether (600 mg, 8.3 mmol), NaHCO 3 (680mg, 8.0mmol), and a solution of commercially available tetrafluoro-2-(tetrafluoro-2-iodoethoxy)ethanesulfonyl fluoride (3.5g, 8.0mmol) of structure 1 in Figure 1 was slowly added to Na 2 S 2 o 4 (1.4 g, 8.0 mmol). The reaction mixture was stirred at 5°C for 50 minutes. The pH of the reaction mixture was adjusted to 6.2-7.0 by the addition of 3.0 N aqueous HCl, and the mixture was stirred at 25°C for an additional 20 minutes. use CH 2 Cl 2 The reaction mixture was extracted, washed with water and concentrated under reduced pressure. The oily residue was dissolved in acetone (38 mL), and the solution was added to stirred 2-methyl-butene-2 (36 mL), NaH 2 PO 4 (4.0g, mmol), NaClO 2 (5.0g, mmol) and water (40mL) mixture. The reac...
Embodiment 2
[0402] This example illustrates the preparation of difluorosulfonic acid linkers and resin-linked difluorosulfonic acids (Figure 2).
[0403] A solution of chloride (3.8 g, 16.8 mmol) of structure 5 of Figure 2 and thiourea (1.3 g, 17 mmol) in EtOH (10 mL) was stirred at 70 °C for 4 h, then cooled to room temperature. A solution of NaOH (1.2 g, 30 mmol) in water (10 mL) was added, the reaction mixture was stirred overnight at room temperature, concentrated under reduced pressure, acidified to pH 6, and washed with CH 2 Cl 2 Extract and wash with water. Dissolve the crude thiol of structure 6 in Figure 2 in CH 2 Cl 2 (30mL), water (30mL) and acetic acid (2mL). The solution was cooled to 0-5 °C, and the Cl 2 Gas was bubbled through the solution for 1 hour. The reaction mixture was concentrated and washed with CH 2 Cl 2 extracted, washed with cold water, dried (MgSO 4 ) and concentrated to give the sulfonyl chloride of Figure 2,7 (4.8 g, 97%). 1 H NMR (CDCl 3 , δ): 1.6...
Embodiment 3
[0409] This example illustrates the use of perfluorosulfonyl fluoride linkers in the deoxygenation of various phenols (Figure 3).
[0410] Phenol (0.68mmol), K 2 CO 3(100 mg, 0.72 mmol), Figure 3, a mixture of the resin-linked linker of structure 4 (80 mg, 0.034 mmol) and DMF (1.0 mL) was shaken overnight at room temperature. Described resin water, DMF and CH 2 Cl 2 Washed, and dried in vacuo overnight to yield the resin-linked phenol of Figure 3, structure 13. Add Pd(OAc) to the dry resin of Figure 3, structure 13 2 (6.0 mg, mmol), 1,3-bis(diphenylphosphino)propane (dppp, 16.0 mg, mmol), DMF (1.2-1.4 mL) and HCO 2 H (180μL) and Et 3 N (460 μL) mixture. The mixture was shaken at 85°C for 120 minutes. Filter the polymer beads and filter with Et 2 O washing. use Na 2 CO 3 The combined organic phases were washed with aqueous solution and water and evaporated to dryness. The residue was dissolved in Et 2 O and short SiO 2 The column was eluted to remove inorganic re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com