Water-soluble matrix for external transdermal paster and preparing method thereof
A technology of water-soluble matrix and transdermal patch, applied in the field of water-soluble matrix of external transdermal patch and its preparation, can solve the problems of high price, poor adhesion, impossibility of popularization and use, etc. The effect of strong sex and reducing individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
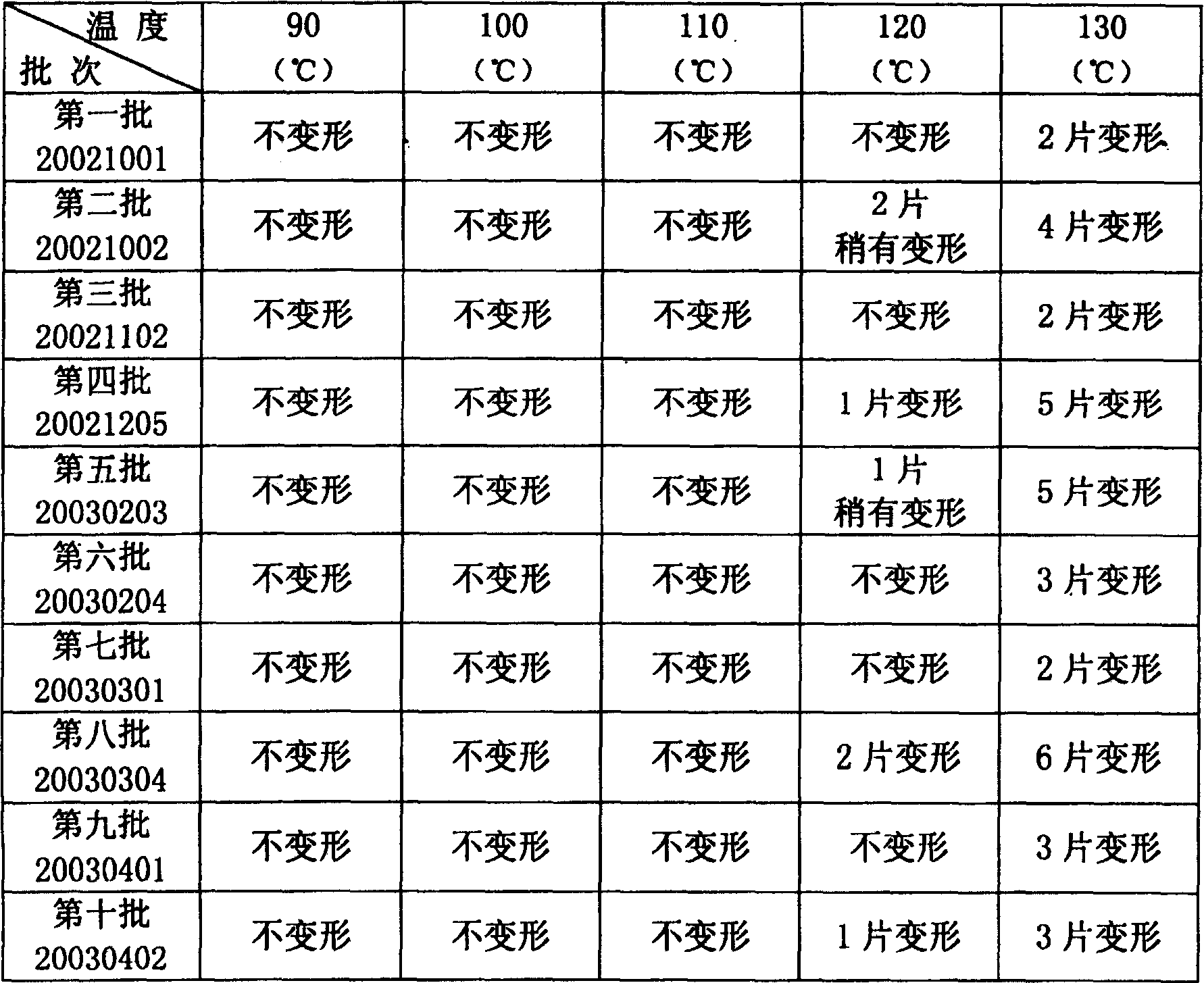
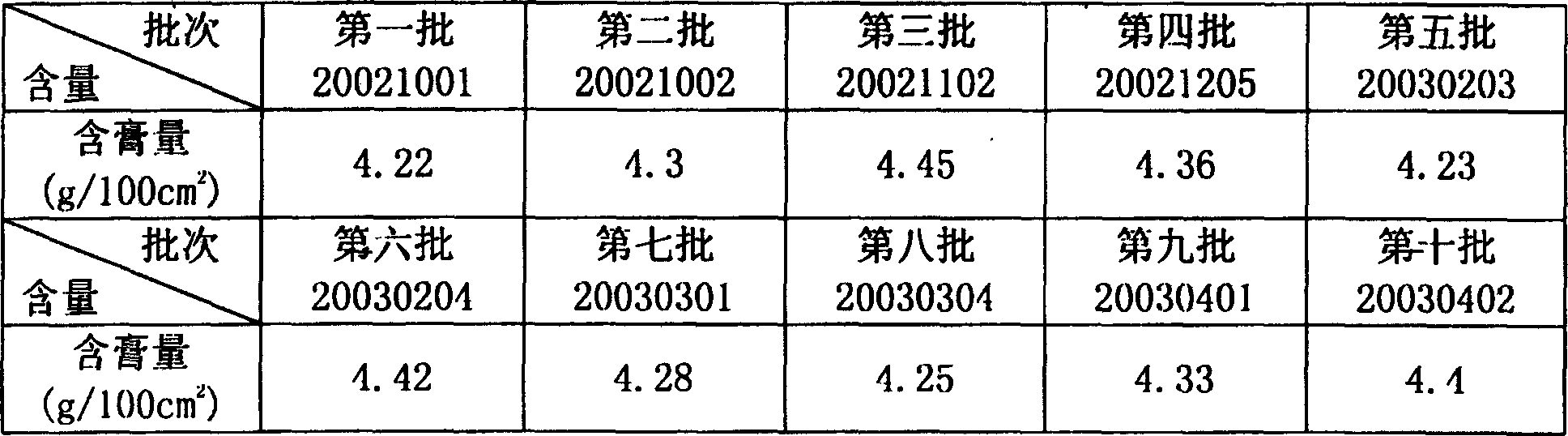
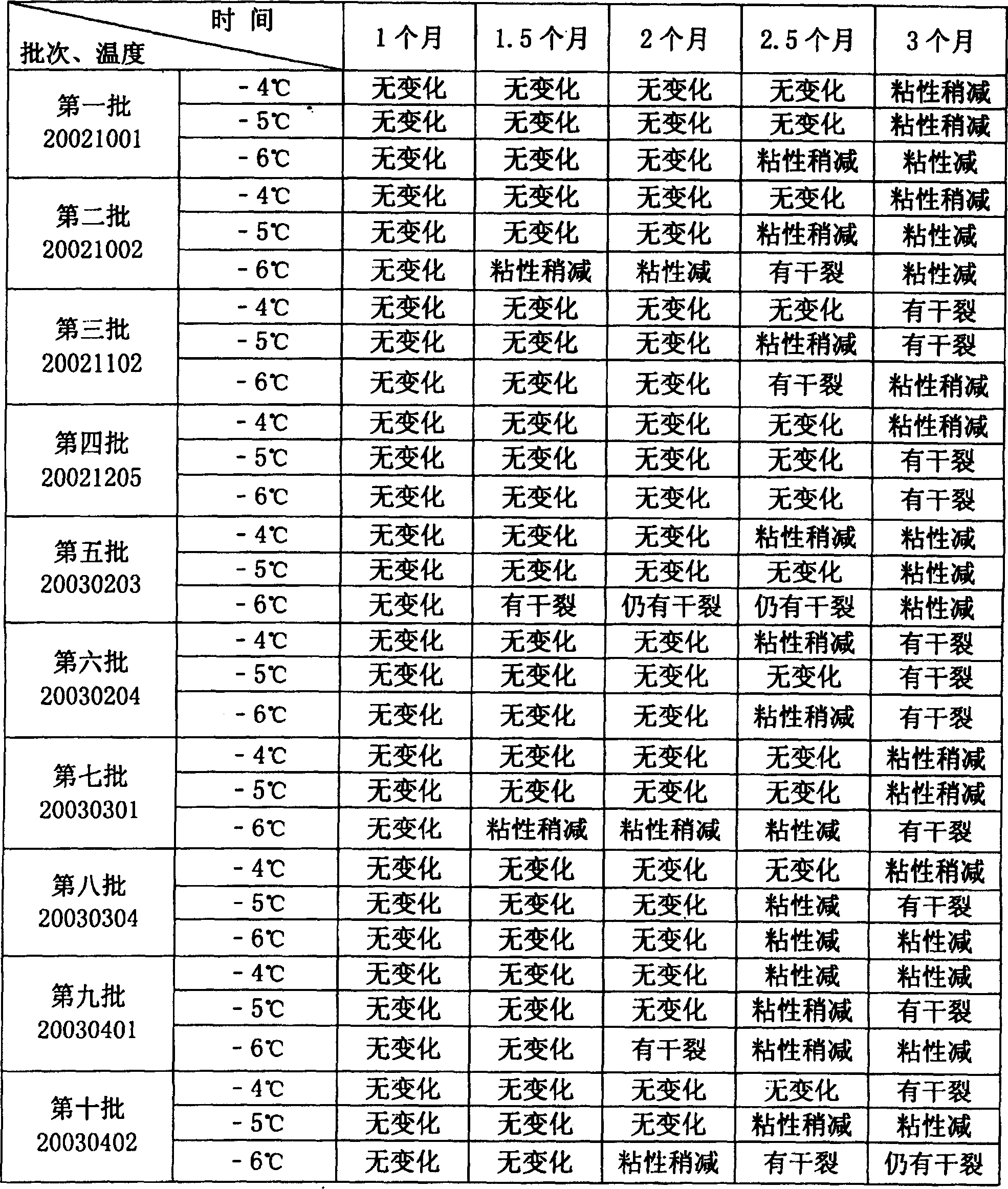
Image
Examples
Embodiment 1
[0035] It is made of the following raw materials in weight ratio: polyacrylic acid and sodium polypropylene copolymer: polyvinylpyrrolidone: polyvinyl alcohol: glycerin: potassium hydroxide: tartaric acid = 3: 2: 2: 15: 0.3: 0.2.
[0036] Its preparation method is:
[0037] a. Get 2kg of polyvinylpyrrolidone to make a 12% aqueous solution;
[0038] b. Get 2kg of polyvinyl alcohol to make a 10% aqueous solution;
[0039] C. get tartaric acid 0.2kg to make content and be the aqueous solution of 0.1%;
[0040] d. Fully mix the prepared polyvinylpyrrolidone aqueous solution, organic acid aqueous solution, enol aqueous solution, polyacrylic acid and polypropylene sodium copolymer 3kg, glycerin 15kg and potassium hydroxide 0.3kg, stir evenly, and place to form a matrix .
Embodiment 2
[0042] It is made of the following raw materials in weight ratio: polyacrylic acid and sodium polypropylene copolymer: polyvinylpyrrolidone: polyvinyl alcohol: glycerin: sodium hydroxide: tartaric acid=12:10:10:75:2.5:1.5.
[0043] The preparation method is the same as in Example 1.
Embodiment 3
[0045] It is made of the following raw materials in weight ratio: polybutacrylic acid and sodium polybutene copolymer: polyvinylpyrrolidone: polyvinyl alcohol: glycerin: potassium hydroxide: tartaric acid = 8: 8: 6: 50: 1: 0.5 .
[0046] Its preparation method is: a. take polyvinylpyrrolidone 8kg to make the aqueous solution that content is 16%;
[0047] B. get polyvinyl alcohol 6kg to make content be the aqueous solution of 16%;
[0048] C. getting tartaric acid 0.5kg to make content is the aqueous solution of 0.5%;
[0049] d. Fully mix the polyvinylpyrrolidone aqueous solution, organic acid aqueous solution, enol aqueous solution, polybutacrylic acid and polybutene sodium copolymer 8kg, glycerin 50kg and potassium hydroxide 1kg prepared above, stir evenly, place, that is into a matrix.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com