Use of IL-18 inhibitors for treatment or prevention of sepsis
An inhibitor, a sepsis technology, applied in the application field of IL-18 inhibitor in the treatment or prevention of sepsis, which can solve the problems of reducing inflammation and blocking the maturation of IL-1β
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
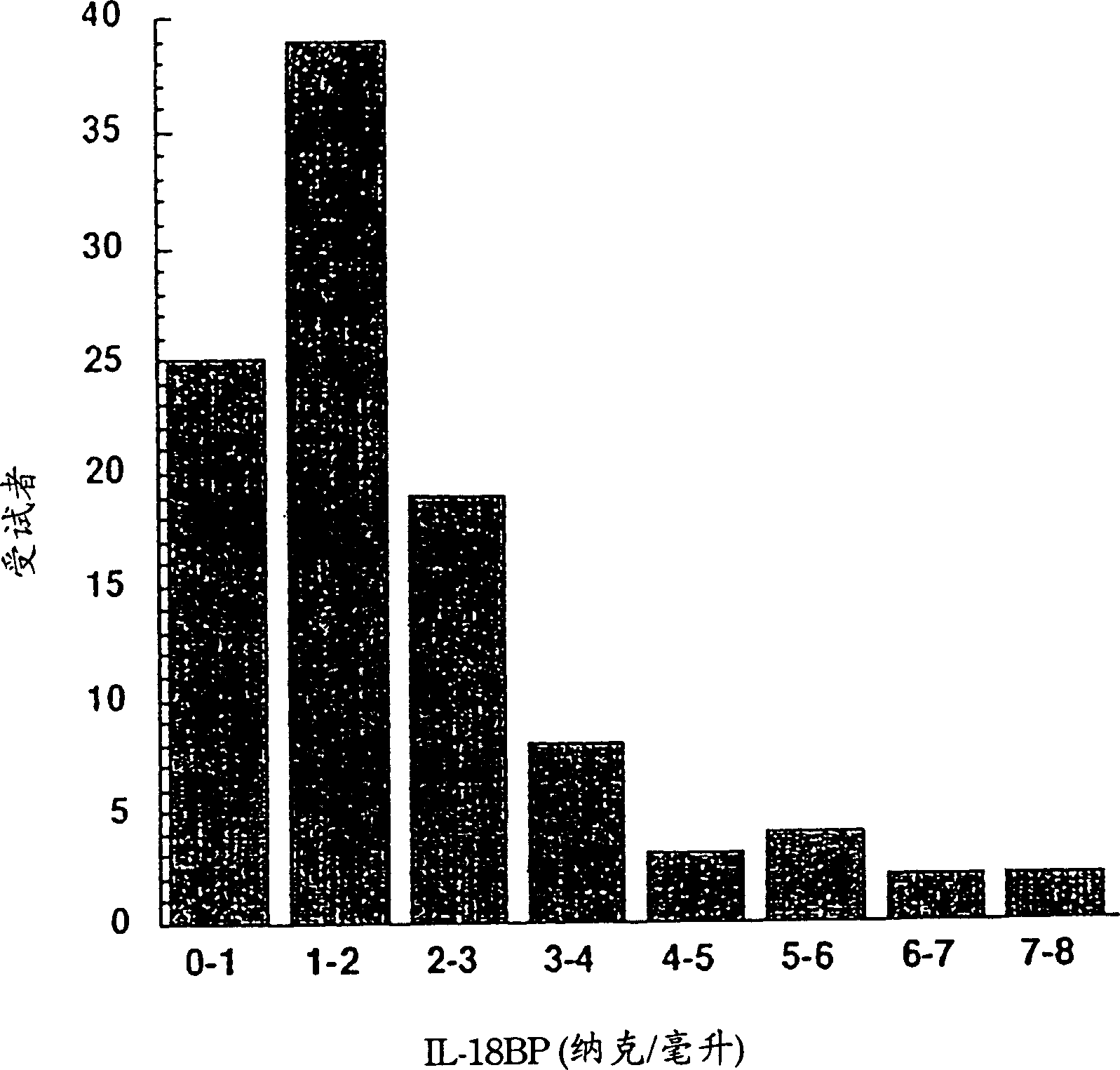
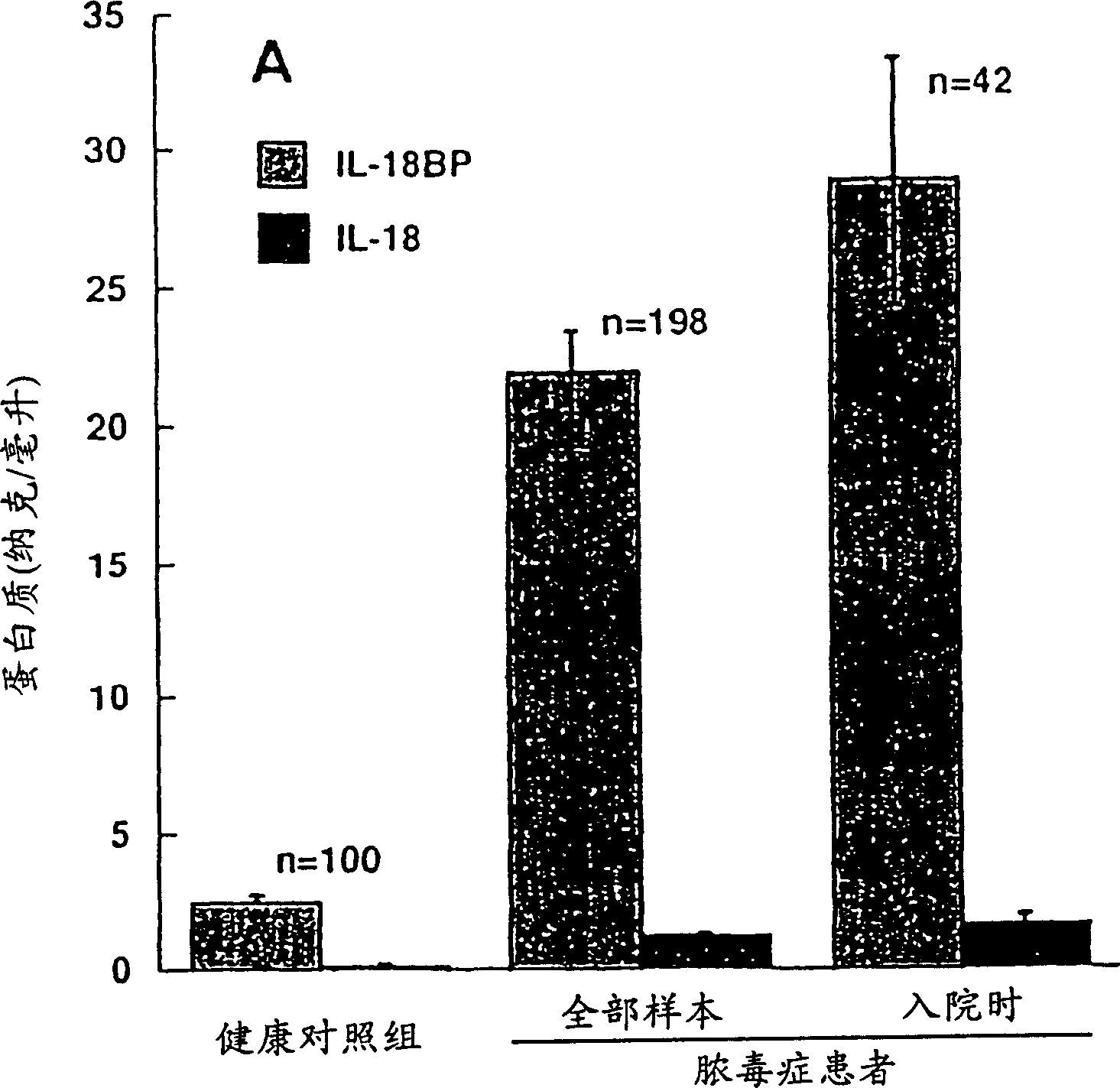
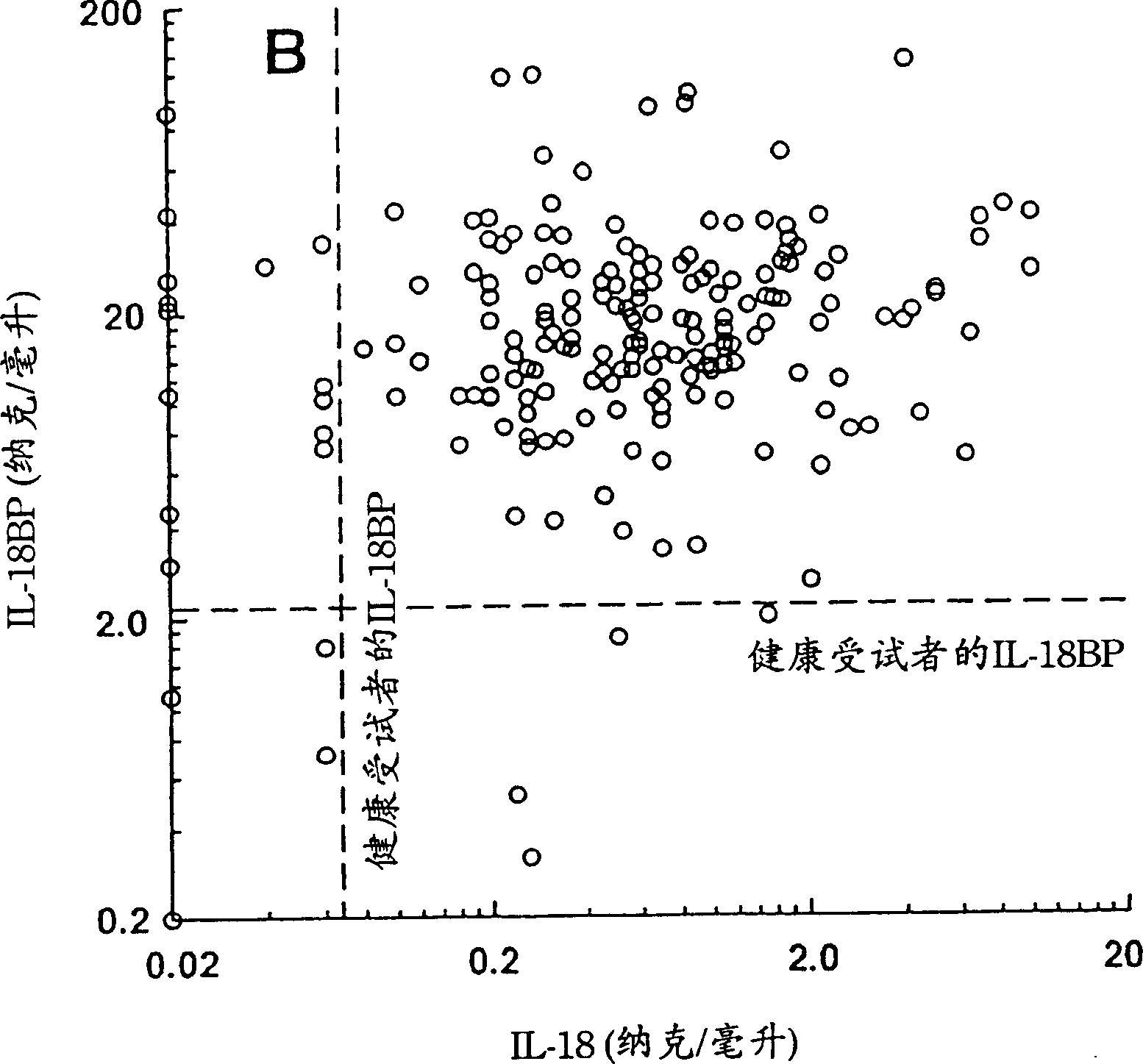
[0166] Example 1 Serum IL-18BPa and IL-18 levels of healthy individuals and patients with sepsis
[0167] Measuring specific circulating cytokines and their natural inhibitors in healthy individuals and patients can yield information about their involvement in disease progression and severity. As mentioned in the Background section, a key regulator of sepsis is IFN-γ. Because IL-18 is a co-inducer of IFN-γ, the levels of IL-18 and its natural inhibitor, IL-18BP splice variant a, were monitored in patients with sepsis and compared with healthy subjects using a specific ELISA assay. The levels found were compared (Example 3).
[0168] IL-18 and IL-18BPa levels in healthy subjects
[0169] The mean IL-18 level in 107 healthy subjects was 64±17 pg / ml measured by electrochemiluminescence (ECL, Pomerantz et al., 2001). The degree of interference of related proteins was tested by ECL method. It was found that it was not affected in any way in the presence of mature IL-1β or proIL-1...
Embodiment 2
[0179] Example 2 IL-18BPa induces production of Staphylococcus epidermidis in cells contained in whole blood samples of healthy subjects
[0180] Effect of IFN-γ
[0181] As mentioned in the Background section, the Gram-positive bacterium Staphylococcus epidermidis is known to cause sepsis. The main cause of this disease is the induction of cytokines such as IL-1, IFN-γ and TNF-α. Since IL-18 is a co-inducer of IFN-γ, the effect of IL-18 inhibition on the induction of IFN-γ production by S. epidermidis was tested. IL-18BPa was used as an inhibitor of IL-18, a histidine-tagged recombinant form produced in Chinese hamster ovary cells.
[0182] Take 0.5 ml of blood and 0.5 ml of RPMI growth medium containing Staphylococcus epidermidis (from ATCC) with IL-18BP or only Staphylococcus epidermidis (Cellgro Mediatech, Hendon.VA, supplemented with 10 mM L-glutamine, 100 U / ml penicillin , 100 μg / ml streptomycin and 10% FBS [Gibco BRL, Grand Island, NY]) were mixed in a 5 ml ...
Embodiment 3
[0186] Example 3 Establishment of IL-18BP-specific ELISA test
[0187] The ELISA assay compared two anti-IL-18BPa antibodies: mouse monoclonal antibody 582.10, which is a subclone of monoclonal antibody 582 described in Example 4, used as a capture antibody, and a rabbit polyclonal antibody for detection (implemented Example 4). ELISA well plates (Maxisorb; Nunc A / S, Roskilde, Denmark) with microtiter 96 wells were coated with anti-IL-18BPa mAb 582.10 (subclone of mAb 582, 4 μg / ml in PBS), overnight at 4 °C. Plates were washed with 0.05% Tween 20 in PBS (wash solution) and blocked (2 hours, 37° C.) with a 1:10 dilution of BSA stock solution / water (KPL, Geithesburg, MD). The BSA stock solution was diluted 1:15 (diluent) with water and used to dilute all test samples and detection antibodies. Serum samples were diluted at least 1:5 with diluent and 100 microliter aliquots were added to the wells. Highly purified human IL-18BPa (made in Chinese hamster ovary cells and purifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com