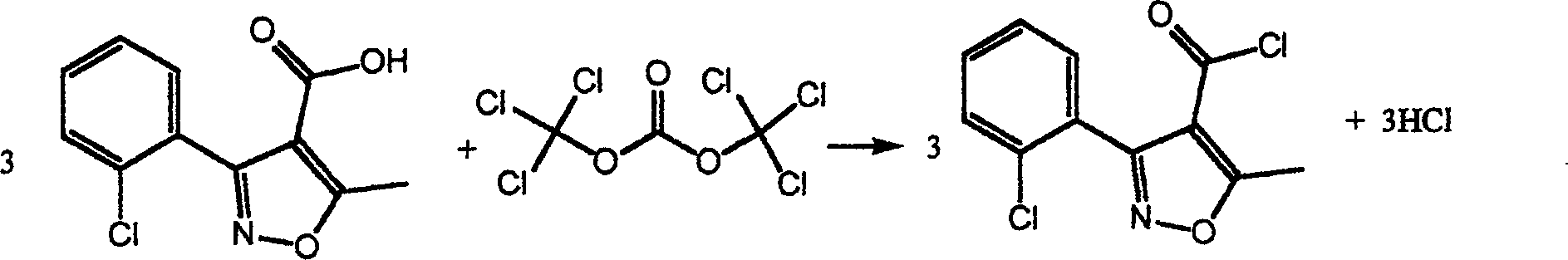
Chemical synthesis method of 3-(2-chlorophenyl)-5-methyl-4-isoxazole formyl chloride
An isoxazolecarbonyl and chemical synthesis technology, which is applied in the field of chemical synthesis of 3-2-chlorophenyl)-5-methyl-4-isoxazolecarbonyl chloride, can solve the problem of poor production safety and serious equipment corrosion , long reaction cycle and other problems, to achieve the effect of low production cost, small equipment corrosion, safe and reliable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] 3-(2-chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid: bis(trichloromethyl) carbonate: tetrabutylurea=1: 0.33: 0.02 (molar ratio), the toluene consumption is 10 times the mass of 3-(2-chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid.
[0013] 3-(2-Chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid, tetrabutylurea and toluene were added to the reaction kettle, after stirring evenly, bis(trichloro Toluene solution of methyl)carbonate, open the hydrogen chloride absorption system at the same time, then raise the temperature to 110°C, reflux for 2 hours, and recover the toluene by distillation under reduced pressure after the reaction, and finally collect the distillate at 130-132°C at 0.667KPa, freeze solidify. The yield is 95.6%, the melting point is 42-43°C, and the content (HPLC) is 99.6%.
Embodiment 2
[0015] 3-(2-chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid:bis(trichloromethyl)carbonate:tetrabutylurea=1:0.5:0.01 (molar ratio), o-dichloro The amount of benzene used is 5 times the mass of 3-(2-chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid.
[0016] Add 3-(2-chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid, tetrabutylurea and o-dichlorobenzene into the reaction kettle, stir evenly, add bis (Trichloromethyl) carbonate tetrahydrofuran solution, open the hydrogen chloride absorption system at the same time, then raise the temperature to 145-150 ° C for 1 hour, after the reaction is complete, first recover o-dichlorobenzene by vacuum distillation, then vacuum distillation, at 0.667KPa The fractions at 130-132°C were collected, frozen and solidified, the yield was 96.0%, the melting point was 41-42°C, and the content (HPLC) was 99.2%.
Embodiment 3
[0018] 3-(2-chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid: bis(trichloromethyl)carbonate: tetrabutylurea=1:0.50:0.01 (molar ratio), recovered o The amount of dichlorobenzene used is 5 times of the mass of 3-(2-chlorophenyl)-5-methyl-4-isoxazole formic acid.
[0019] 3-(2-Chlorophenyl)-5-methyl-4-isoxazolecarboxylic acid, tetrabutylurea and recovered o-dichlorobenzene were added to the reaction kettle, and after stirring evenly, drop it within 45 minutes at room temperature Add the o-dichlorobenzene solution of two (trichloromethyl) carbonates, and the others are the same as in Example 2. The yield is 96.6%, the melting point is 41-42°C, and the content (HPLC) is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com