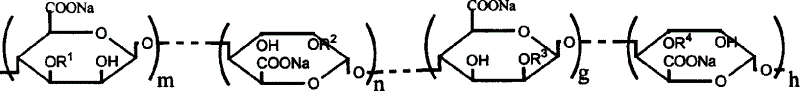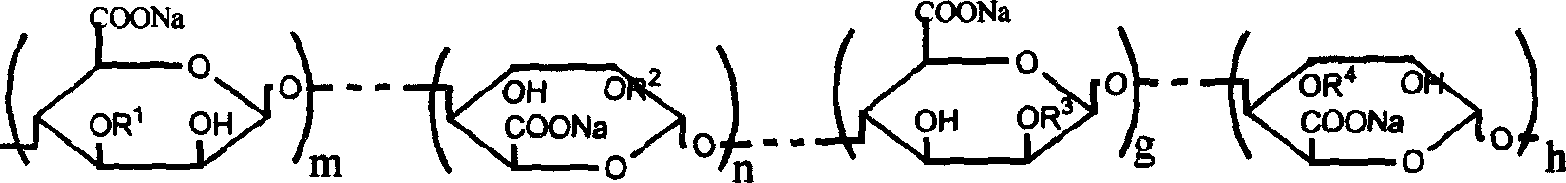Sodium alginate sulfuric ester and preparation method and use thereof
A technology of sodium alginate and sulfuric acid ester, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, extracellular fluid diseases, etc., and can solve problems such as high prices and insufficient sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 10g of sodium alginate containing 80ml of formamide and 20ml of ClSO 3 In the sulfation reagent of H, stir at 60°C for 4 hours to obtain a brown-red solution, add an appropriate amount of deionized water, dialyze for 3 days, dissolve and shrink to dryness to obtain 15 g of sulfated sodium alginate. The sample S% is 13.21%, C%, H% are 21.05% and 1.93%, respectively. The degree of substitution of sulfate ester groups was calculated to be 1.41 / uronic acid unit (assuming that both sulfate and carboxyl groups form sodium salts). Infrared detection appears 1250cm -1 and 800cm -1 The vibration peak of the sulfate ester group. Permeation gel chromatography test has a retention time of 7.51 minutes, and the test conditions are: gel column TSKG3000-PW, mobile phase 0.1mol / L NaCl aqueous solution, flow rate 1.0mL / min, column temperature 30°C, and the refractive index detects the sample. The concentration of the sample is 0.4%, which is calibrated by the Pullulan standard s...
Embodiment 2
[0023] Add 10g sodium alginate containing 80ml formamide and 4mlClSO 3 In the sulfation reagent of H, stir at 40°C for 4 hours to obtain a brown-red solution, add an appropriate amount of deionized water, dialyze for 3 days, dissolve and shrink to dryness to obtain 12 g of sulfated sodium alginate. The sample S% is 5.69%, C%, H% are 30.78% and 3.03%, respectively. The degree of substitution of sulfate ester groups was calculated to be 0.41 / uronic acid unit (assuming that both sulfate and carboxyl groups form sodium salts). Infrared detection appears 1250cm -1 and 800cm -1 The vibration peak of the sulfate ester group. Permeation gel chromatography test has a retention time of 7.21 minutes, and the test conditions are: gel column TSKG3000-PW, mobile phase 0.1mol / L NaCl aqueous solution, flow rate 1.0mL / min, column temperature 30°C, and the refractive index detects the sample. The concentration of the sample is 0.4%, which is calibrated by the Pullulan standard sample provid...
Embodiment 3
[0025] Add 10g of sodium alginate containing 80ml of formamide and 30ml of ClSO 3 In the sulfation reagent of H, stir at 60°C for 4 hours to obtain a brown-red solution, add an appropriate amount of deionized water, dialyze for 3 days, dissolve and shrink to dryness to obtain 15 g of sulfated sodium alginate. The sample S% is 14.28%, C%, H% are 19.75% and 1.90%, respectively. The degree of substitution of sulfate ester groups was calculated to be 1.63 / uronic acid unit (assuming that both sulfate and carboxyl groups form sodium salts). Infrared detection appears 1250cm -1 and 800cm -1 The vibration peak of the sulfate ester group. Permeation gel chromatography test has a retention time of 7.71 minutes, and the test conditions are: gel column TSKG3000-PW, mobile phase 0.1mol / L NaCl aqueous solution, flow rate 1.0mL / min, column temperature 30°C, and the refractive index detects the sample. The concentration of the sample is 0.4%, which is calibrated by the Pullulan standard s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com